Acute heart failure is a major and growing public health problem worldwide with high morbidity, mortality, and cost. Despite recent advances in pharmacological management, the prognosis of patients with acute decompensated heart failure remains poor. Consequently, nonpharmacological approaches are being developed and increasingly used. Such techniques may include several modalities of ventilation, ultrafiltration, mechanical circulatory support, myocardial revascularization, and surgical treatment, among others. This document reviews the nonpharmacological approach in acute heart failure, indications, and prognostic implications.
Keywords
Acute heart failure (AHF) constitutes a clinical condition with a complex pathophysiology, defined as a heterogeneous syndrome of signs and symptoms of new-onset or gradual/rapidly worsening heart failure (HF), requiring urgent intervention.1 Despite recent advances in pharmacological management, its morbidity and mortality remain high. Consequently, nonpharmacological approaches are being developed and increasingly used.
POSITIVE AIRWAY PRESSURE THERAPYPositive airway pressure (PAP) therapy has emerged as an important tool in the treatment of several forms of acute respiratory failure, representing a valuable nonpharmacological tool in the management of AHF. This therapy involves the maintenance of PAP through invasive or noninvasive methods.
In the clinical context of HF, PAP has several effects on hemodynamics: a) systemic venous return reduction and right ventricular unloading by increasing intrathoracic pressure,2 and b) changes in total pulmonary vascular resistance, which is the major determinant of right ventricular afterload.3 Total pulmonary vascular resistance is characterized by a U-shaped curve according to lung volume variation (the lowest pulmonary vascular resistance can be observed in the lung volume around functional residual capacity).
Positive airway pressure also has several effects on the respiratory system in HF: a) alveolar recruitment and prevention of alveolar collapse, improving gas exchange and oxygenation; b) induction of fluid shifts back from the alveoli and the interstitial space to the pulmonary circulation, and c) reduction of the respiratory muscle load and work of breathing.4
Types or Modes of Positive Airway Pressure Therapy in Heart Failure TreatmentSeveral types or modes of PAP therapy can be considered for HF. All of them apply PAP, in particular positive end-expiratory pressure, each type with different purposes. The main available types of PAP for AHF management are presented below.
Invasive VentilationIf invasive ventilation is required, lung protective modes should be performed to prevent pulmonary injury.
Noninvasive VentilationNoninvasive positive pressure ventilation (NPPV) has been widely used and its use should be encouraged to alleviate signs and symptoms of respiratory distress due to cardiogenic pulmonary edema. Evidence to date on the potential benefit of NPPV is derived from case series and relatively small, randomized, controlled trials. Most compare continuous PAP (CPAP) or bilevel PAP with standard therapy and suggest that noninvasive ventilation improves symptoms and physiological variables, reducing rates of invasive ventilation and mortality in selected patients.5–8 Currently, there is still uncertainty regarding the real prognostic impact of NPPV in the context of AHF.
A recent randomized clinical trial of patients with AHF showed that neither type of NPPV reduced short-term mortality or the rate of endotracheal intubation when compared with standard therapy, despite early improvements in symptoms and in surrogate measures of disease severity.9 However, a recent Cochrane review revealed lower mortality and reduced intubation rate with the use of in-hospital NPPV compared with standard medical treatment alone in patients with AHF.10
The question remains whether therapy with NPPV in AHF is of more benefit in patients with systolic dysfunction. Some authors argue that in patients with predominantly diastolic dysfunction (who require a relatively high filling pressure), the effects of positive pressure therapy might compromise venous return.11
Continuous Positive Airway PressureThis therapy is the most widely used mode of PAP therapy in patients with HF. It provides a constant level of positive pressure to maintain airway patency during spontaneous breathing.
Observational studies on the effects of positive pressure on cardiac physiology in the setting of acute pulmonary edema have shown that CPAP improves cardiac output and lung compliance, decreasing lung and airway resistance.12 Some studies have also shown that CPAP in patients with AHF reduces sympathetic tone, blood pressure, and heart rate.13 When high-quality, randomized clinical trials were pooled in a meta-analysis, treatment with CPAP was associated with a 26% lower intubation rate and a trend toward overall lower mortality.14
Bilevel Positive Airway PressureBilevel PAP provides 2 fixed levels of PAP: a higher level of pressure during inspiration (inspiratory PAP) and a lower level of pressure during expiration (expiratory PAP). Its major difference compared with CPAP is that it provides pressure support (difference between inspiratory PAP and expiratory PAP) during inspiration.
The level of inspiratory PAP plays an important role in unloading respiratory muscles, reducing the work of breathing, controlling obstructive hypopnea, maintaining alveolar ventilation, and reducing PaCO2 (partial pressure of carbon dioxide).
Expiratory PAP produces hemodynamic and respiratory effects similar to those provided by CPAP. A retrospective analysis15 reported that the use of bilevel PAP in patients with acute pulmonary edema was associated with a low intubation rate and intensive care unit stay.
However, theoretical advantages over CPAP have not been demonstrated in some nonrandomized comparisons with AHF patients, suggesting a possible negative effect.16 Studies comparing the use of bilevel PAP vs CPAP in acute pulmonary edema reported that bilevel PAP was better than CPAP in increasing the oxygenation, decreasing carbon dioxide and respiratory rate and improving symptoms.17,18 However, a recent randomized clinical trial showed no differences in treatment efficacy or safety between the 2 noninvasive ventilation treatments.9 More comparative clinical trials are needed to answer these questions and close monitoring and proper patient selection are essential.
European guidelines generally recommend the use of NPPV therapy in AHF patients with a respiratory rate > 20 breaths/min and signs of pulmonary edema without shock (recommendation IIa B).19
Several factors have been reported to be associated with the success of noninvasive ventilation: a) patient-ventilator synchrony; b) Glasgow coma score > 9; c) acceptance of the technique by the patient; d) small amount of secretions; e) APACHE II (Acute Physiology and Chronic Health Evaluation II) score < 21; f) hypercapnia; g) initial arterial pH > 7.1; h) adequate response in the first hour of treatment, and i) high blood pressure at baseline.
Withdrawal is usually progressive, in general under the following conditions: a) improvement of dyspnea, without the use of accessory muscles; b) heart rate < 100 bpm; c) respiratory rate < 30breaths/min; d) FiO2 (fraction of inspired oxygen) ≤ 50%, spontaneous breathing without NPPV, SaO2 (arterial oxygen saturation) > 90% and comfortable patient, and e) PaO2 (partial pressure of oxygen) > 70mmHg or PaO2/FIO2 ratio > 200mmHg.
Contraindications and Possible Complications of Noninvasive VentilationThere is no clear consensus on absolute and relative contraindications for the use of NPPV,20 some of them being described as exclusion criteria in many studies. For possible contraindications and complications see Table 1.
Contraindications and Possible Complications of Noninvasive Positive Pressure Ventilation
| Absolute contraindications |
| Inability to protect airway |
| Pneumothorax |
| Inability to remove secretions |
| Significant facial fractures/anatomical abnormalities |
| Active gastrointestinal or upper airway bleeding |
| Recent gastrointestinal or upper airway surgery |
| Hemodynamic instability |
| Relative contraindications |
| Nausea and vomiting |
| Agitation |
| Cardiac ischemia or acute myocardial infarction |
| Significant chest trauma |
| Severe hypoxemia (PaO2/FiO2 < 75) |
| Possible complications |
| Patient discomfort |
| Intolerance to the technique: asynchrony |
| Injuries to the nasal bridge |
| Claustrophobia |
| Dry mucous membranes |
| Gastric distension |
| Hypotension |
FiO2, fraction of inspired oxygen; PaO2, partial pressure of oxygen.
Congestion and fluid retention, the hallmark of HF, cause about 90% of HF hospitalizations,21 with their severity being associated with worse outcomes. The presence of overt congestion characterizes patients with higher neurohumoral activation, particularly of the renin-angiotensin-aldosterone system, intrarenal microvascular and cellular dysregulation, and oxidative stress. In these patients, glomerular filtration rate is usually decreased, sodium reabsorption in the proximal tubule is increased, and urinary sodium excretion is reduced.
The adverse effect of persistent congestion on outcomes has been shown in several studies. In the ESCAPE study, elevated pulmonary capillary wedge pressure was one of the strongest independent predictor in post-discharge mortality in patients with AHF.
Increased central venous pressure is independently associated with renal impairment, worsening renal function, and unfavorable outcomes.23 The increased amounts of sodium and water reabsorbed by the kidney as a result of enhanced systemic and intrarenal neurohumoral activity predominantly fill the compliant venous circulation, increasing central venous pressure and intraglomerular pressure, further impairing filtration rate.24
The optimal method for achieving successful decongestion while minimizing changes in renal function and neurohumoral activation remains an area of intensive ongoing research. Furthermore, successful treatment of congestion cannot simply be translated into renal or clinical improvement.
Intravenous loop diuretics remain the first-line therapy for AHF.22 Notwithstanding the widespread use of these agents, there are persistent uncertainties about the safety profile and appropriate dosing. Importantly, diuretic resistance and worsening renal function can be relevant issues in patients presenting with AHF.25 Approximately 40% of hospitalized HF patients are discharged with unresolved congestion,26 denoting increased rehospitalization and mortality rates.27
Peripheral veno-venous ultrafiltration, a mechanical strategy to remove fluid, has emerged as an alternative/complementary modality to diuretic therapy in AHF presenting with systemic and pulmonary congestion. The ultrafiltration procedure uses a transportable ultrafiltration console along with a disposable extracorporeal blood circuit. It allows effective removal of sodium and water across a semipermeable membrane in response to a transmembrane gradient driven by the hydrostatic pressure difference. Solutes with smaller size with respect to membrane pores, such as electrolytes and urea contained in that amount of plasma water, are removed at the same concentration of the plasma water. However, neither significant correction of electrolyte and metabolic disturbances nor a significant removal of high molecular weight substances (eg, myocardial-depressant factors and cytokines) can be expected from isolated ultrafiltration because of its operational characteristics. Thus, ultrafiltrate is isotonic, whereas the urinary output with loop diuretics is predominantly hypotonic, minimizing the electrolyte abnormalities or neurohumoral activation. Ultrafiltration removes more sodium (and less potassium) than diuretics for an equivalent volume loss.28 Thereby, favorable effects are not reproduced by the removal of an equivalent fluid volume by high-dose intravenous diuretic.
Ultrafiltration may contribute to the clinical short-term improvement of congestive HF patients via several pathways related to the heart/lung interaction: a) direct mechanical action achieved via the correction of fluid overload, which reduces right atrial pressure, pulmonary artery pressure, pulmonary vascular resistances, and ventricular filling pressures; b) reduction in cardiac edema, which improves diastolic function and overall cardiac performance; c) correction of the neurohumoral imbalance and relative arterial underfilling component induced by HF and diuretic therapy, and d) reduction in extravascular lung water, shunt effect and dead space, with improved gas exchange and oxygenation.29,30
The mechanisms underlying the long-term positive effect of ultrafiltration remain unclear. It is likely that differences in neurohumoral response to fluid withdrawal might play a role, despite a similar capacity of ultrafiltration and diuretic therapy to improve symptoms and promote short-term clinical stabilization in patients without refractory HF. Indeed, the decongestion obtained with ultrafiltration, compared with diuretic therapy, does not elicit, or even turns off, neurohumoral compensatory mechanisms, resulting in long-term maintenance of the clinical benefit and, consequently, in a reduction of further hospitalizations.31
The question is when patients with congestion may benefit the most from ultrafiltration, and when they should be treated with this approach as a first-line therapy. Besides the timing of ultrafiltration initiation, another important factor is the filtration rate, since volume removal exceeding the plasma refill rate may lead to intravascular volume depletion, hypotension, and renal hypoperfusion. Efforts should be made to identify the potential subpopulations most likely to benefit from ultrafiltration. In this regard a recent study prospectively compared AHF patients treated with ultrafiltration stratified by left ventricular ejection fraction. No differences were observed between groups in therapeutic responses, overall length of stay, or in-hospital mortality.32 The findings of that study suggested that ultrafiltration might be safely applied to congestive patients regardless of the ejection fraction.
Overall, there is a scarcity of vigorous clinical trials in the field of ultrafiltration therapy for AHF. Most of studies are small-sized, retrospective, without controls and with short follow-up. In additions, the differences in HF patient populations and ultrafiltration methodology hamper comparison of the results. Nevertheless, the evidence suggests that ultrafiltration improves pulmonary and peripheral congestion, lung function, and hemodynamics without adverse effects on renal function. Favorable hemodynamic changes with ultrafiltration may result in improved renal function and restoration of diuretic responsiveness.33,34
The RAPID-CHF trial34 was the first randomized controlled trial of ultrafiltration for AHF. Forty patients were randomized to a single session of ultrafiltration or to usual medical care. For the primary endpoint of weight loss at 24hours, there was no significant difference between groups. However, fluid removal after 24hours and dyspnea and HF symptoms at 48hours were significantly improved with ultrafiltration.
The UNLOAD trial,35 the largest in this field, randomized 200 patients with AHF to either ultrafiltration (primary therapy) or loop diuretic therapy within 24hours of admission. Patients with severe renal dysfunction and those with hemodynamic instability were excluded. The primary endpoints were weight loss, dyspnea relief at 48h and readmission rate. The ultrafiltration group had greater weight loss but there was no difference in patient-reported dyspnea. Notably, the UNLOAD trial showed a decrease in rehospitalization for HF with ultrafiltration compared with diuretic therapy. This secondary efficacy endpoint, however, was based on a relatively small number of events. Of note, there was a trend toward worsening renal function in the ultrafiltration group.
In a retrospective comparison of 25 ultrafiltration, 25 intravenous diuretics, and 25 nesiritide treated patients, those treated with ultrafiltration had the greatest increase in blood urea and creatinine. Despite worsening renal function, all-cause 30-day rehospitalizations were fewer in the ultrafiltration treated patients (12% ultrafiltration vs 24% diuretics vs 28% nesiritide).36 These findings suggest that decongestion of patients with AHF may improve outcomes even when intense diuresis produces deterioration in renal function.
In the CUORE trial,37 patients randomized to ultrafiltration had a significantly lower frequency of rehospitalization for congestive HF than did control subjects and this beneficial effect was maintained for up to 1 year. The reduction in rehospitalizations was associated with maintenance of a more stable body weight and renal function and lower diuretic dose in the first 6 months after discharge compared with controls. These reductions, as in the UNLOAD trial, confirm that congestion represents one of the major prognostic determinants and also suggest that decongestion alone is not sufficient, given the same degree of fluid removal in both groups.
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure (CARRESS-HF) trial,38 the second largest multicenter study in this field, randomized patients who also presented with cardiorenal syndrome and persistent congestion to receive either an algorithm-based pharmacologic regimen or ultrafiltration as a rescue therapy. The primary bivariate endpoint was the change in serum creatinine level and body weight at 96hours after enrollment. Patients in the ultrafiltration group did not show greater weight loss and had a significant increase in serum creatinine level and a higher rate of serious adverse events such as sepsis. Due to a lack of evidence of benefit for ultrafiltration as well as an excess number of adverse events, the enrollment ended sooner than planned. Nevertheless, the study arms were not totally comparable: a) the use of inotropes and vasodilators was prohibited in the ultrafiltration group, whereas they were allowed in the pharmacologic care arm after 48hours according to urine output, and b) the diuretics dose was adjusted in the pharmacologic therapy group whereas the ultrafiltration rate was uniformly delivered. The findings of the CARRESS-HF and other similar studies39 raised questions regarding the role of ultrafiltration after the development of diuretic resistance, progressive renal dysfunction, and cardiorenal syndrome.
Nevertheless, some points should be highlighted regarding the latter 2 studies. First, clinical indications for ultrafiltration and the HF population studied were different, with ultrafiltration being an elective first-line treatment in CUORE and a rescue therapy in HF complicated by acute kidney injury in CARRESS-HF. Second, the decongestion protocol used in ultrafiltration-treated patients in the CARRESS-HF was not customized and was probably too aggressive in terms of speed and the total amount of fluid removal. Third, the use of a stepped pharmacologic therapy in the control group of the CARRESS-HF, including vasodilators and inotropic agents, aimed to maintain a predefined urine output target. The addition of adjuvant therapies in the ultrafiltration group was prohibited unless they were deemed to be necessary as rescue therapy. Fourth, in CARRESS-HF, as well as in the UNLOAD study, loop diuretics were discontinued or prohibited during ultrafiltration.
The safety and efficacy of ultrafiltration depend upon the ability to remove fluid without causing hemodynamic instability and/or worsening renal function. To achieve this goal, the amount and rate of fluid removal must be clearly established. If ultrafiltration rates are too high, hemodynamic instability occurs because the refilling of the intravascular space from the interstitium cannot compensate for the reduction in intravascular volume. Lower ultrafiltration rates give rise to gradual intravascular refilling from the interstitial space that reduces extracellular fluid without inducing intravascular volume depletion. In practice, ultrafiltration should initially be prescribed at low ultrafiltration rates (100-200mL/h). Rates of ultrafiltration exceeding 250mL/h are no longer recommended. Of note, patients with predominantly right-sided HF or patients with HF and preserved systolic function indicate that these patients are especially prone to intravascular volume depletion. Therefore, all patients should be carefully evaluated and their clinical status monitored while undergoing ultrafiltration. Monitoring the changes in hematocrit via online hematocrit sensors can be used as surrogate markers for plasma refill rate. Echocardiographic monitoring of the changes in the inferior vena cava and left atrial diameters, impedance analysis and biomarkers are among other potential options.
Complications of UltrafiltrationRandomized data on potential adverse events associated with ultrafiltration are suboptimal. CARRESS-HF showed that the incidence of serious adverse events was significantly higher in the ultrafiltration arm compared with the pharmacological arm (72% vs 57%; P=.03), being mainly attributed to HF and renal failure. High ultrafiltration rates and highly negative fluid balance are not technically but clinically limited. Individual tolerance to fluid removal during isolated ultrafiltration depends on the complex interplay of several factors influencing the vascular refilling rate. As a general rule, an aggressive approach to weight change by isolated ultrafiltration might negatively impact on both systemic hemodynamics and, consequently, renal function.
Apart from renal and hemodynamic complications, isolated ultrafiltration shares problems that are typical of any other renal replacement therapies techniques based on extracorporeal circulation, the most common being those linked to catheter use (eg, venous access-related infection) and circuit clotting. Hemolysis and hemorrhage are rare complications.
Ultrafiltration has emerged as an attractive option for AHF management, based on the recent advances in our understanding of the mechanisms underlying heart disease and cardiorenal syndrome as well as suboptimal efficacy and safety of conventional therapies. However the overall results introduce a new level of complexity regarding the relationships between fluid removal strategies, renal function, and outcomes. Future studies are needed to further define the role of ultrafiltration therapy in this setting.
MECHANICAL CIRCULATORY SUPPORTAcute heart failure comprises a wide spectrum of clinical conditions including cardiogenic shock (CS), a state of end-organ hypoperfusion caused by ventricular dysfunction, based on clinical and hemodynamic criteria, associated with an overall poor prognosis. There are multiple causes for CS but myocardial infarction with left ventricular failure remains the most common. The general rationale for using mechanical circulatory support (MCS) in patients with CS is restoring adequate systemic perfusion pressure, gaining time to ventricular recovery. Over the past decades, innovation in this field has changed CS management.
To date there is no universal consensus on indications for MCS in AHF, the following being the most common: a) postinfarction CS; b) fulminant myocarditis; c) acutely decompensated chronic HF unresponsive to inotropic agents; d) inability to wean from cardiopulmonary bypass after cardiac surgery; e) graft failure after heart transplantation, and f) post-cardiac arrest. In those settings, the role of MCS can be the following:
- •
Bridge to transplantation.
- •
Destination therapy: an alternative for patients contraindicated for a heart transplantation.
- •
Bridge to recovery.
- •
Bridge to bridge: for those patients who present with severe shock or following cardiac arrest and are supported with a temporary device to evaluate if they could become candidates for a long-term support.
- •
Bridge to decision: used when the best option for a given patient is unclear at the time of device implantation.
The ideal device should enable both hemodynamic support and myocardial protection. Available devices for MCS are: intra-aortic balloon pump (IABP), extracorporeal life support (ECLS) and ventricular assist devices (VADs). Current European guidelines recommend considering the use of a percutaneous assist device for MCS in refractory CS to provide for a quick and easy initiation, with no preference for device selection (recommendation IIa C).40 A detailed description of different assistance devices is beyond the scope of this article and has been reviewed elsewhere.41–43 Therefore, only major considerations are covered here. Currently, data derived from randomized clinical trials on the effectiveness and safety, indications, and optimal timing of each device are limited.
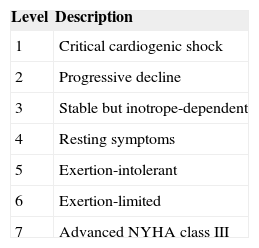
The major determinants of success in the field of MCS for HF are patient selection (often based on subjective criteria) and timing of device implantation (dualistic effect of an early use-balance between efficacy and device-related complications). INTERMACS (Interagency Registry for Mechanically Assisted Circulatory Support), a United States registry that acquires data on patients supported with Food and Drug Administration-approved few registry, patients in New York Heart Association III to IV class are classified in 7 clinical profiles according to their signs and symptoms (Table 2), to define clinically important differences in the severity of disease among patients with advanced HF.44
Interagency Registry for Mechanically Assisted Circulatory Support Clinical Profiles
| Level | Description |
|---|---|
| 1 | Critical cardiogenic shock |
| 2 | Progressive decline |
| 3 | Stable but inotrope-dependent |
| 4 | Resting symptoms |
| 5 | Exertion-intolerant |
| 6 | Exertion-limited |
| 7 | Advanced NYHA class III |
NYHA, New York Heart Association.
Nearly 70% of all registered VADs have been implanted in the sickest subset of patients with HF (INTERMACS profiles 1 and 2). However, low INTERMACS profiles have been consistently associated with higher perioperative mortality.45 In the few past years, the field has changed Most patients are now receiving temporary percutaneous circulatory support, as a way to support circulation and triage for eventual durable VAD.
To assess which form of MCS is best suited for each patient, several factors should be considered in the initial strategy:
- •
Etiology of cardiac dysfunction
- •
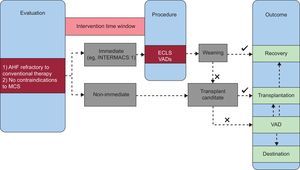
Time frame for implantation: if immediate action is required (eg, INTERMACS 1 patients), surgically implanted VADs are ruled out. In such cases, IABP, ECLS or percutaneous implantable VADs are the options. IABP is reserved for “less severe” forms, with a mean arterial pressure above 40mmHg. In more advanced forms, percutaneously implanted VADs are able to support the circulation until completely replacing cardiac function. When pulmonary support is also needed, ECLS is the only option available.
- •
Projected time course of recovery
- •
Long-term VAD or transplant eligibility
- •
Ventricular function reserve
- •
Arterial access and vessel size
- •
Severity of pulmonary dysfunction
The Figure presents a simplified algorithm for the management of patients with AHF when MCS is considered.
Proposed algorithm for the management of patients with acute heart failure when mechanical circulatory support is considered. AHF, acute heart failure; ECLS, extracorporeal life support; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; MCS, mechanical circulatory support; VAD, ventricular assist device.
The IABP is the most current and less expensive form of MCS. It functions as a volume displacement device. The alternated inflation and deflation of the balloon, synchronized with the cardiac cycle, improve peak diastolic pressure and coronary blood flow while reducing aortic pressure, afterload, and myocardial oxygen consumption. It is easy and fast to deploy and requires some cardiac function to be effective.
Before the advent of the IABP-SHOCK trial,46 conceived to finally support a contemporary clinical practice with evidence, any recommendation for IABP treatment in ST-segment elevation myocardial infarction with CS was based on pathophysiological considerations and expert opinion. The results showed no benefit from use of IABP on 30-day mortality or in any key secondary endpoints. It was a multicenter, open-label, randomized trial, which enrolled 600 participants from 37 centers. The patients, presenting with acute myocardial infarction complicated by CS, were randomized to either IABP or no IABP. More than 95% underwent primary percutaneous coronary intervention, with stent placement in 90%. At 30 days, there was no significant difference in the primary outcome of mortality and none in the secondary endpoints between IABP and controls. Six- and 12-month follow-ups were carried out in the IABP-SHOCK II47 trail, showing no long-term benefit. IABP was not associated with any significant increase in adverse events, including reinfarction, stent thrombosis, bleeding, sepsis, or stroke. A further important limitation is that the overall mortality rate of patients enrolled in the study was lower (40% vs 42-48%) compared with other registries, suggesting more mild or moderately severe shock cases. The authors point out that the high use (90%) of catecholamines actually reflected the severity of the CS in the study. It is true, however, that such high use of catecholamines might have offset the potential benefits of IABP. Also, IABP should have been used as the primary therapy in the IABP group, rather than as an additional therapy to catecholamines. To finish, timing of IABP insertion was left to the discretion of the investigator: in the vast majority of cases, it was inserted post-PCI. After the trial results were published, the European Society of Cardiology 2012 ST-segment elevation myocardial infarction guidelines downgraded the indication to IABP support in postinfarction CS from IC to IIb B. In summary, there is no evidence or recommendations for the use of IABP in CS patients.
Extracorporeal Life SupportExtracorporeal life support in the venoarterial configuration can completely replace cardiopulmonary function and is indicated for severe forms of CS and refractory cardiac arrest. Blood is withdrawn via the venous system (usually the femoral vein or right atrium) and pumped through an oxygenator, where gas exchange of oxygen and carbon dioxide takes place. It is then returned to the arterial system (usually the femoral artery or ascending aorta). For patients with renal insufficiency, a hemofiltration system may be integrated into the circuit. It requires careful monitoring of anticoagulation and is conceived for short or mid-term support, carrying a not negligible rate of complications (typically, bleeding at the site of cannulation or lower limb ischemia), which rises exponentially with the duration of support.
Due to the theoretical advantage of prompt cannulation and support almost anywhere, it is adequate for emergency settings. Although offering an effective first-line assistance to circulation for patients in cardiac arrest and refractory CS, the capacity of peripheral ECLS to left ventricle assistance is limited. In the presence of severely reduced left ventricular function, significant afterload mismatch and consequently inadequate ventricular decompression may occur. Moreover, blood flow still returns to the left atrium from the bronchial circulation. The increase in wall stress compromises myocardial perfusion and enhances oxygen consumption, reducing the likelihood of ventricular recovery.48 Elevated ventricular and left atrial pressures can induce severe pulmonary edema. To avoid this, some centers use an IABP in conjunction with peripheral ECLS to reduce left ventricular afterload and pulmonary congestion. In this regard, a recent study49 evaluated 253 patients undergoing ECLS for CS, 24% with concomitant IABP (IABP group). Successful ECLS weaning rate was significantly higher in the IABP group than in the control group (61.7% vs 42.0%; P=.008). However, there was no significant difference in in-hospital mortality between the 2 groups (68.6% vs 72.0%; P=.58).
There are some other methods available for ventricular discharge in patients on peripheral ECLS (eg, left ventricular apical or percutaneous trans-septal left atrial cannulas connected to the venous line via a Y-shaped connector) promoting pulmonary decongestion and ventricular recovery.
Ventricular Assist DevicesVentricular assist devices are continuous flow pumps connected to the patient's circulation that replace the function of the left heart, right heart, or both. Pumps may be centrifugal or axial. Percutaneously implanted VADs (intravascular or extracorporeal) are intended for temporary, short-term use, while surgically implanted (intracorporeal or paracorporeal, axial or centrifugal) VADs are for mid- or long-term use. Thus, the latter are not the first choice for MCS in AHF. Recently published International Society for Heart and Lung Transplantation Guidelines for MCS provide recommendations for long-term MCS options for patients with advanced HF.50
Many types of VADs are available, with different implantation sites and techniques. The technology for VADs has undergone tremendous improvements, which have improved outcomes for HF patients.
In the setting of AHF or in refractory CS, a bridge-to-decision device needs to be easy to insert, provide biventricular support when needed, and generate sufficient flow. It is now understood that an implantable, long-term left VAD, although an excellent therapy for chronic HF, s not usually an appropriate choice in this context. Short-term, percutaneously implanted VADs, which generally consist of internal cannula and an external pump, have been developed for this application and have become a widely accepted treatment option.51
The 2014 European Society of Cardiology and the European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularization52 recommend left VAD implantation in younger patients with no contraindication for cardiac transplantation as a bridge to transplantation and in patients not eligible for transplantation as a bridge to recovery or with the goal of destination therapy. However, there is a lack of definitive evidence regarding its routine application. Three randomized trials and a large registry have demonstrated superior hemodynamic support with percutaneous MCS systems than with IABP, with no differences in mortality but with an increased risk of adverse events.53–56
The Impella-EUROSHOCK57 registry constitutes to date the largest work investigating emergency support with the Impella-2.5 device for treatment-refractory CS and included 120 patients. All patients had refractory post-myocardial infarction CS and received temporary circulatory support with the Impella-2.5-percutaneous left-VAD. The primary endpoint was mortality at 30 days, whereas secondary endpoints were parameters of device efficacy and safety. Thirty-day mortality was 64.2%, probably due to the use of the device in critically ill patients, and 44.5% of patients were successfully weaned. After VAD implantation, a significant decrease of lactate levels was recorded and the incidence of major complication was negligible.
Contraindications for Mechanical Circulatory SupportIn general, patients with irreversible neurological damage, untreated malignant disease or with a life expectancy shorter than 1 year are contraindicated for MCS. Other contraindications are specific for each device and depend essentially on technical aspects.
CARDIAC TRANSPLANTATIONCardiac transplantation is considered the gold standard for the treatment of refractory end-stage HF. Since the first successful heart transplantation in 1967, significant improvements have been made regarding donor and recipient selection, surgical techniques, and postoperative care. It provides significantly increased survival, exercise capacity, and quality of life compared with conventional treatment. However, the number of potential organ donors has not changed, despite a growing number of patients on the waiting list. To overcome this issue, the United Network for Organ Sharing implemented an allocation system to prioritize the sickest patients on the list to receive organs.
We refer to recommendations for patient selection for heart transplantation that are endorsed by most societies in the field.58 Transplantation can be considered as an option in severe AHF known to have a poor outcome. However, transplantation is not possible until the patient's condition has been stabilized. In this setting, MCS have an essential role, as noted above.
MYOCARDIAL REVASCULARIZATIONRecommendations for management of patients with AHF in the setting of acute coronary syndromes57 states that emergency myocardial revascularization (percutaneous or surgical) is indicated for patients with ST-segment elevation myocardial infarction or CS due to non–ST-segment elevation myocardial infarction (recommendation IB). In the subpopulation of patients with CS complicating myocardial infarction, current guidelines encourage multivessel percutaneous coronary intervention of all critical stenosis or highly unstable lesions in addition to the culprit lesion (recommendation IIa B).
Application of early revascularization has markedly increased in clinical practice. However, rates are still unsatisfactory, ranging from 50% to 70% in registries.59
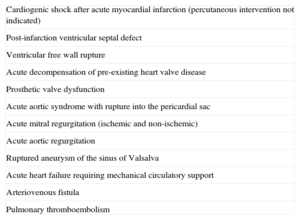
SURGICAL TREATMENTSurgical intervention is indicated in some causes of AHF (Table 3). Surgical options include coronary revascularization, correction of the anatomic lesions, valve replacement or reconstruction, as well as MCS. Coronary angiography is usually performed because it is believed that concomitant revascularization may improve prognosis.
Cardiovascular Causes of Acute Heart Failure Requiring Surgical Approach
| Cardiogenic shock after acute myocardial infarction (percutaneous intervention not indicated) |
| Post-infarction ventricular septal defect |
| Ventricular free wall rupture |
| Acute decompensation of pre-existing heart valve disease |
| Prosthetic valve dysfunction |
| Acute aortic syndrome with rupture into the pericardial sac |
| Acute mitral regurgitation (ischemic and non-ischemic) |
| Acute aortic regurgitation |
| Ruptured aneurysm of the sinus of Valsalva |
| Acute heart failure requiring mechanical circulatory support |
| Arteriovenous fistula |
| Pulmonary thromboembolism |
Pacing and electrical cardioversion are recommeded in patients hemodynamically compromised by severe bradycardia or tachydysrhythmia, respectively, to improve clinical condition (recommendation IC).
CONCLUSIONSIn summary, nonpharmacological management of AHF is increasingly used to complement intravenous therapies. Patients with AHF and no hemodynamic instability may benefit from NPPV. There are very few contraindications and the nurse workload is minimal. In patients with a high level of congestion, recent increase in body weight and diuretic resistance, all associated with a low cardiac output state but no signs of shock, ultrafiltration might be of a great benefit. Although the use of IABP in CS lacks evidence, the use of ECLS and VAD should be encouraged as early as possible to limit the use of catecholamines, which should be administered at the lowest dose and for the shortest duration possible.
CONFLICTS OF INTERESTNone declared.