Multidisciplinary strategies for the management of heart failure (HF) improve outcomes. We aimed to evaluate the effectiveness of noninvasive home telemonitoring in ambulatory patients with HF already included in a structured multidisciplinary HF program.
MethodsProspective intervention study with before/after comparison design of an interactive telemedicine platform in HF patients, randomized 1:1 into two groups: A) Motiva System with educational videos, motivational messages, and questionnaires, and B) Motiva System+self monitoring of blood pressure, heart rate, and weight. Hospitalizations were compared over 12 months prior to and post study inclusion. Quality of life was evaluated using the generic EuroQoL visual analogue scale and the specific questionnaire Minnesota Living With Heart Failure Questionnaire.
ResultsThere were 92 patients included (71% male; 66.3±11.5 years; 71% ischemic aetiology). During real-time telemonitoring over 11.8 months (interquartile range, 8.6-12), 14,730 questionnaires were administered with 89% median response rate. Hospitalizations for HF decreased by 67.8% (P=.010) and for other cardiac causes by 57.6% (P=.028). The number of days in hospital for HF decreased by 73.3% (P=.036), without statistically significant differences between groups, and for other cardiac causes by 82.9% (P=.008). The perception of quality of life improved significantly both for the generic scale (P<.001) and for the HF specific questionnaire (P=.005).
ConclusionsHF patients who used an interactive telehealth system with motivational support tools at home spent less time in hospital and felt their quality of life had significantly improved. No significant differences were observed between groups.
Keywords
Heart failure (HF) is a disease with a poor prognosis and high rate of hospitalization and readmission.1,2,3 Health education, adequate pharmacological treatment, close monitoring by health care providers, and self monitoring are key in preventing rehospitalization.4 It is also well known that patients with HF have a diminished quality of life (QoL) for various reasons (eg, dietary restrictions, adverse effects of multiple medications, limited socialization due to symptoms). Through effective implementation of the guidelines approved by scientific societies for diagnosis and management of HF, and by offering comprehensive disease management strategies, specific HF units or disease management programs have had a great impact on the reduction of hospital admissions for HF and on improvement of patient QoL.5,6 Our previous experience concurs with those found in the literature, with a 49% reduction in HF hospital admissions during the first year after the set-up of a structured multidisciplinary HF unit.7
However, readmission rates remain high for this patient population, and novel options based upon the use of information and communication technologies to manage chronic disease need to be evaluated and implemented. Of these new technologies, remote telemonitoring and telephone support programs have been the most widely explored.8,9 Noninvasive home telemonitoring has the dual advantage of providing continuous monitoring while encouraging patient participation in self-management of the disease. Several studies have already demonstrated that remote telemonitoring may have an impact on disease-associated morbidity.10 Interest in telemonitoring is growing, and recent technological advances as well as the development of a more user-friendly computer interface may lead to increased patient satisfaction and acceptance of these new technologies.
The objective of this study was to evaluate the impact of a telemedicine program with specifically designed educational videos as a main new feature combined with questionnaires, messages, and self-monitoring equipment on patients attending a structured multidisciplinary HF unit. Number of hospitalizations and days in hospital for HF and other cardiac causes 12 months prior to enrollment was compared with the number of hospitalizations and days in hospital during the follow-up period. Additionally, our goal was to analyze changes in patients’ perceived QoL using a generic scale and a specific questionnaire for HF at the outset and at the end of the study. Finally, we aimed to compare the use of a telemedicine system with and without self monitoring.
Methods Design and Study PopulationWe conducted a prospective intervention study with before/after comparison design of an interactive telemedicine platform in a multidisciplinary HF program of a university hospital. This is a structured unit receiving two thirds of its patients from the cardiology department and the rest from internal medicine and emergency departments. All patients are followed up at regular predefined intervals, with additional visits as required in case of decompensation, and all provided written informed consent for the analysis of clinical data for research purposes. The regular schedule of visits include a minimum of quarterly visits with nurses, biannual visits with physicians, and elective visits with geriatricians, psychiatrists, and rehabilitation physicians. A weight chart is kept by each patient and reviewed at every visit. Face-to-face education is reinforced with printed leaflets for patients and relatives, and with posters in the waiting room that alert patients to the signs of worsening HF.11
Consecutive outpatients attended in the HF unit between July 2007 and December 2008 were invited to participate in the study. Inclusion criteria were: a) age > 18 years; b) New York Heart Association functional class II–IV; c) permanent residence (the patient's or the home of a family member/friend); d) in-home access to a television; and e) understanding and being able to adequately carry out self monitoring at home. Patients with a life expectancy of less than 1 year, patients participating in another study, or those who did not give consent to participate in this study were excluded. The study patients were assigned to 2 groups, A and B, following 1:1 simple and blinded randomization. Group A utilized the Motiva System (educational videos, motivational messages, questionnaires) with no self-monitoring equipment; Group B utilized the Motiva System+self-monitoring tools (Motiva Plus).
The study complies with the requirements of the Declaration of Helsinki, and was approved by the hospital's ethics committee. All participants gave their written consent.
Upon enrollment, the following information was collected and recorded: a) demographic characteristics, baseline clinical status, and treatment; b) hospitalizations in the previous year and number of days in hospital for HF and other cardiac causes; c) physical examination data; d) patients’ perception of QoL using the EuroQoL visual analogue scale (EQ-VAS)12 and the Minnesota Living With Heart Failure Questionnaire (MLWHFQ)13 (the EQ-VAS scale scores 0-100, being 0 the worst and 100 the best perception of QoL, while the MLWHFQ scores 0-105, being 0 the best and 105 the worst perception of QoL); and e) knowledge of the disease and degree of compliance in blood pressure and weight self-monitoring, as well as other measures of health education. After the initial visit, a remote telemonitoring system was installed in the home of study participants.
All patients continued their routine follow-up visits at the unit at the established intervals and at the end of the study. Hospitalizations and length of stay within the 12 months prior to enrollment and during the 12-month follow-up period utilizing telemedicine were compared. Furthermore, QoL and the usability of the system were evaluated at enrollment and after 12 months. Hospitalization data were obtained reviewing the electronic clinical history from the Catalan Institute of Health.
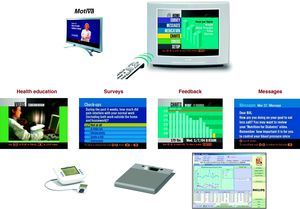
Remote Telemonitoring SystemThe Motiva telemedicine system (Philips) is an interactive platform that transmits data via a broadband Internet connection from the patient's home (using the patient's television) to a workstation at the hospital's HF unit. The system allows for sending information from the medical staff performing the telemonitoring, which was displayed in Spanish on the patient's television (educational videos, questionnaires to establish the patient's baseline status, personalized messages and alarms) and deployment of automated self-monitoring equipment (scale and sphygmomanometer) in the patient's home to record weight, heart rate, and blood pressure; these data are presented graphically on the patient's television and are transmitted, analyzed, and presented to the health care staff supporting the patient via a dedicated web application (Figure 1).
Figure 1. Telemonitoring tools used through a patient's television. The bottom line shows the telemonitoring equipment for weight and blood pressure and a graph of vital signs, as visualized at the heart failure unit workstation.
Patients randomized to the self-monitoring group were instructed to record their weight, blood pressure, and heart rate each morning before breakfast.
Patients were sent 20 videos covering topics such as: disease overview (symptoms, daily care, etiology), medication (changes in therapy, compliance), doctors’ visits, relapse prevention, cholesterol management, hypertension, lifestyle (physical activity, family and friends, emotional control, travel, stress), nutrition (eating out, liquids, low salt diet), vaccinations, and alcohol consumption. In addition, patients were given 18 videos containing real-life patient interviews.
Questionnaires were sent to patients periodically at pre-established times, using their television: weekly (edema), every 2 weeks (fatigue and new symptoms, or compliance in arterial hypertension and diabetes), monthly (changes in medication or hospital visits), or quarterly (MLWHFQ and EQ-VAS).
Statistical AnalysisTo compare changes in the number of hospitalizations and days in hospital between the two study groups, a sample size of 58 patients was necessary for a 50% reduction in one group and a 25% reduction in the other, with 5% and 20% alpha and beta errors, respectively. This sample (116 patients) allowed for estimation of a total prevalence of 50% with 10% accuracy. The sample was oversized in 80% to prevent dropouts and rejections.
Data were compared between groups using the χ2 test for categorical variables and Student's t-test for continuous variables; nonparametric analyses were used for non-Gaussian variables. Comparison of data before and after inclusion in the telemedicine program was carried out using the Student's t-test for paired data. The Wilcoxon test for paired data was used to compare QoL questionnaires. All of the comparisons were bilateral and performed with an alpha error of 5%. The Stata 11 statistical package was used for data analysis.
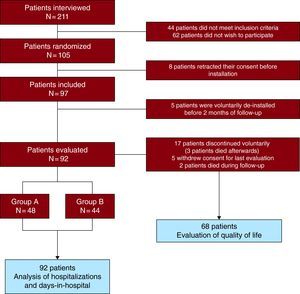
ResultsWe interviewed 211 consecutive patients, of whom 44 (20.8%) did not meet the inclusion criteria and 62 (29.4%) did not wish to participate in the study (Figure 2). Of the 105 patients that initially consented to participate in the study, 13 retracted their consent before installation of the telemedicine system (8 patients) or less than 2 months after installation (5 patients), a telemonitoring time considered too short to have an impact on study variables, and were excluded from the analysis (Figure 2). Therefore, 92 patients (71% male), with a mean age of 66.3±11.5 years, were included in the study. Forty-eight patients were randomized into Group A and 44 into Group B. Patients’ demographic and clinical characteristics were similar between groups, as shown in Table 1. There were no differences between initial and final doses of betablockers and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers.
Figure 2. Study flow chart.
Table 1. Demographic Characteristics, Baseline Clinical Status, and Treatments.
| Total | Group A (Motiva) | Group B (Motiva Plus) | P value | |
| Patients | 92 | 48 | 44 | |
| Age (years) | 66.3±11.5 | 66.0±12.1 | 66.7±11.0 | .76 |
| Age>70 years | 37 (40.2%) | 20 (41.7%) | 17 (38.6%) | .77 |
| Female sex | 27 (29.3%) | 10 (20.8%) | 17 (38.6%) | .06 |
| Primary or lower educational level | 63 (68.5%) | 34 (70.8%) | 29 (65.9%) | .62 |
| Etiology | ||||
| Ischemic heart disease | 65 (70.6%) | 37 (77.1%) | 28 (63.6%) | .16 |
| Idiopathic dilated cardiomyopathy | 13 (14.1%) | 5 (10.4%) | 8 (18.2%) | .37 |
| Hypertensive heart disease | 2 (2.2%) | 1 (2.1%) | 1 (2.3%) | 1.00 |
| Valvular | 5 (5.5%) | 1 (2.1%) | 4 (9.1%) | .19 |
| Others | 7 (7.6%) | 4 (4.2%) | 3 (2.3%) | .78 |
| Time of HF in months | 64±52 | 62±45 | 67±60 | .62 |
| LVEF | 36%±14% | 37%±14% | 35%±13% | .52 |
| Functional class | .79 | |||
| II | 75 (81.5%) | 40 (83.3%) | 35 (79.5%) | |
| III | 17 (18.5%) | 8 (16.7%) | 9 (20.5%) | |
| Comorbidities | ||||
| Hypertension | 52 (56.5%) | 28 (58.3%) | 24 (54.5%) | .71 |
| Diabetes mellitus | 39 (42.4%) | 20 (41.7%) | 19 (43.2%) | .88 |
| COPD | 16 (17.4%) | 7 (14.6%) | 9 (20.5%) | .58 |
| Peripheral vascular disease | 13 (14.1%) | 10 (20.8%) | 3 (6.8%) | .07 |
| Therapy | ||||
| ACE/ARB | 79 (85.9%) | 40 (83.3%) | 39 (88.6%) | .47 |
| Beta blockers | 86 (93.5%) | 45 (93.8%) | 41 (93.2%) | .91 |
| Spironolactone/Eplerenone | 42 (45.7%) | 21 (43.8%) | 21 (47.7%) | .70 |
| Loop diuretic | 75 (81.5%) | 37 (77.1%) | 38 (86.4%) | .25 |
| Digoxin | 32 (34.8%) | 15 (31.3%) | 17 (38.6%) | .46 |
| Statins | 61 (61.3%) | 33 (68.8%) | 28 (63.6%) | .60 |
| Anticoagulants | 31 (33.7%) | 15 (31.3%) | 16 (36.4%) | .60 |
| ASA | 50 (54.3%) | 27 (56.3%) | 23 (52.3%) | .70 |
| CRT±ICD | 20 (21.7%) | 14 (29.1%) | 6 (13.6%) | .07 |
| Hospitalizations in previous year | ||||
| HF | 28 | 10 | 18 | .21 |
| Cardiac: no HF | 26 | 17 | 9 | .19 |
| Days in hospital in previous year | ||||
| HF | 259 | 91 | 168 | .30 |
| Cardiac: no HF | 387 | 131 | 256 | .21 |
ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction.
Data are expressed as mean ± standard deviation or n (%).
Real-time remote home telemonitoring management was achieved for 11.8 months (interquartile range 8.6-12). Seventeen patients (9 in Group A and 8 in Group B) wished to discontinue the telememonitoring program after a mean 5.1±2.3 months of follow-up (3 of them died after voluntary discontinuation of the telemonitoring program). Two other patients died during the evaluation period (Figure 2). The main causes for discontinuation were patient rejection of the telemonitoring program and technical incidents related to the system. Four patients lived alone and none discontinued the program. During the follow-up period, 14,730 questionnaires were sent, with a median response compliance of 89% (interquartile range 58%-99%). There were no differences (P=.54) in response compliance between groups A (median 87.2% [interquartile range 58.3-98.2]) and B (median 91.2% [interquartile range 55.5-99.4]). Self-monitoring compliance for patients randomized to Group B was 76%±19% for weight and 71%±22% for blood pressure. In 80% or more days of telemonitoring, 51.1% and 44.2% of patients monitored weight and blood pressure, respectively. During follow-up, 283 cardiologist visits and 474 nurse visits were performed, with significant differences between groups A and B (108 vs 175, P=.001; and 197 vs 277, P=.002, respectively).
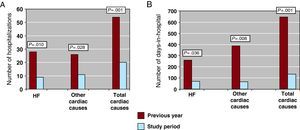
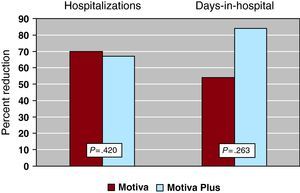
The number of hospitalizations for HF decreased from 28 to 9 (2 were in home care) (67.8%, 95% confidence interval: 58.2%-77.4%, P=.010) and for other cardiac causes from 26 to 11 (57.6%, 95% confidence interval: 47.4%-67.8%, P=.028) (Figure 3A). The number of days in hospital for HF also decreased significantly, from 259 to 69 (73.3%, 95% confidence interval: 64.2%-82.4%, P=.036), and for other cardiac causes from 387 to 66 days (82.9%, 95% confidence interval: 75.2%-90.6%, P=.008) (Figure 3B). The relative reductions in hospital admissions for HF were not significantly different between groups (70% vs 67% for groups A and B, respectively; P=.420) (Figure 4). However, in terms of absolute change, only patients from Group B showed a significant reduction, from 18 to 6 (P=.027). When the number of days in hospital are considered, again the change, assessed as relative reduction between groups, did not achieve statistical significance (53.8% vs 83.9% in groups A and B, respectively, P=.263) (Figure 4); as with the number of hospitalizations, the number of days in hospital for HF decreased significantly only in Group B (from 168 to 27, P=.037).
Figure 3. A: Hospitalizations for heart failure and for other cardiac causes the year prior to enrollment and during follow-up. B: Days in hospital for heart failure and other cardiac causes the year prior to and during follow-up. HF, heart failure.
Figure 4. Number of hospitalizations and days in hospital for heart failure in the previous year and during follow-up in groups A (Motiva) and B (Motiva Plus=Motiva+self-monitoring tools for blood pressure, weight, and heart rate).
Hospitalizations for other cardiac causes decreased from 17 to 6 in Group A (64.7%; P=.026) and from 9 to 5 (44.4%; P=.4) in Group B. The differences between the 2 groups were not significant (P=.35). The length of hospital stay for other cardiac causes decreased similarly in both groups: from 132 to 21 days in Group A (83.9%) and from 256 to 45 days (82.4%) in Group B (P=.33).
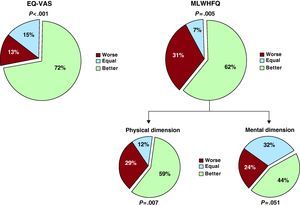
Changes in the perception of QoL were analyzed in patients that attended the final visit and completed the final questionnaires. At the outset of the study, the scores obtained were 47.0±24.5 for the EQ-VAS scale and 12.5 [interquartile range 5-25] for the MLWHFQ. In the final visit, the scores were 65.0±15.7 for the EQ-VAS scale and 8.5 [interquartile range 4-15] for the MLWHFQ. Figure 5 shows the improvement in the perceived QoL in 72% (95% confidence interval: 61.2%-82.8%, P<.001) of the patients for the EQ-VAS scale (P<.001) and in 62% (95% confidence interval: 50.4%-73.6%, P=.005) of the patients for the MLWHFQ. Upon analyzing the physical and mental dimensions of the MLWHFQ data, we found that the most significant improvement occurred in the physical dimension (P=.007; Figure 5). There were no statistically significant differences in QoL improvement between Groups A and B: 61.7% and 82.3% of patients improved in the EQ-VAS scale (P=.165) and 64.7% and 58.8% in the MLWHFQ (P=.690), respectively.
Figure 5. Quality of life scores (final scores compared with initial scores) for EuroQoL visual analogue scale and Minnesota Living With Heart Failure Questionnaire. EQ-VAS, EuroQoL visual analogue scale; MLWHFQ, Minnesota Living With Heart Failure Questionnaire.
Although not an objective of this before/after comparison study, we were able to compare the outcome between participants and nonparticipants for the 75 patients who refused the invitation to participate in the study (including the 5 patients that dropped out of the study within the first 2 months). As shown in Table 2, the 2 groups were similar in demographics, clinical status and therapy, with only a slight increase in ischemic etiology among participants (P=.049). Nonparticipants had fewer hospitalizations for HF during the previous year (28 vs 9; P=.054), but the number of hospital admissions in this group increased during the study period by 55%, as well as the number of days in hospital, which rose by 30.9%. The differences between groups were clearly significant for the number of hospitalizations (P=.006) and borderline significant for days in hospital (P=.075). The mortality rate was almost 2-fold higher among nonparticipants, yet not statistically significant (5.4% vs 9.3% in participants and nonparticipants, respectively; P=.33), likely due to the small number of cases.
Table 2. Patient Demographics, Baseline Clinical Status, and Treatments.
| CARME Study Participants | CARME Study Non Participants | P | |
| Patients | 92 | 75 | |
| Age (years) | 66.3±11.5 | 68.9±17 | .19 |
| Age>70 years | 44 (47.8%) | 44 (58.7%) | .16 |
| Female sex | 27 (29.3%) | 23 (30.7%) | .85 |
| Etiology | |||
| Ischemic heart disease | 65 (70.6%) | 42 (56.0%) | .049 |
| Idiopathic dilated cardiomyopathy | 13 (14.1%) | 9 (12.0%) | .69 |
| Hypertensive cardiopathy | 2 (2.2%) | 4 (5.3%) | .28 |
| Valvular | 5 (5.5%) | 7 (9.4%) | .33 |
| Others | 7 (7.6%) | 13 (17.3%) | .054 |
| Time of HF (months) | 64±52 | 76±61 | .18 |
| LVEF | 36%±14% | 40%±15% | .08 |
| Functional class | .53 | ||
| II | 75 (81.5%) | 61 (81.3%) | |
| III | 17 (18.5%) | 13 (17.3%) | |
| IV | 0 (0.0%) | 1 (1.3%) | |
| Comorbidities | |||
| Hypertension | 52 (56.5%) | 36 (48.0%) | .27 |
| Diabetes mellitus | 39 (42.4%) | 27 (36.0%) | .40 |
| COPD | 16 (17.4%) | 20 (26.7%) | .21 |
| Peripheral vascular disease | 13 (14.1%) | 11 (14.7%) | .92 |
| Therapy | |||
| ACE or ARB | 79 (85.9%) | 65 (86.7%) | .73 |
| Beta blocker | 86 (93.5%) | 65 (86.7%) | .14 |
| Spironolactone/Eplerenone | 42 (45.7%) | 24 (32.0%) | .07 |
| Loop diuretic | 75 (81.5%) | 61 (81.3%) | .98 |
| Digoxin | 32 (34.8%) | 17 (22.7%) | .09 |
| Statins | 61 (61.3%) | 44 (58.7%) | .31 |
| Anticoagulants | 31 (33.7%) | 20 (26.7%) | .33 |
| ASA | 50 (54.3%) | 42 (56.0%) | .83 |
| CRT±ICD | 20 (21.7%) | 11 (14.7%) | .24 |
| Hospitalizations in previous year | |||
| HF | 28 | 9 | .054 |
| Cardiac: no HF | 26 | 17 | .56 |
| Days in hospital in previous year | |||
| HF | 259 | 113 | .27 |
| Cardiac: no HF | 387 | 128 | .09 |
ACE, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor antagonists; ASA, acetylsalicylic acid; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction.
Data are expressed as mean±standard deviation or n (%).
This study shows that a telehealth system combining self monitoring with motivational support tools and added to comprehensive multidisciplinary HF care is able to reduce the number of hospitalizations and days in hospital both for HF and other cardiac causes in HF patients. This result is especially relevant taking into account that HF units already reduce the number of hospitalizations7,14 and the effect of new tools, in this case remote management, provided to patients that already received multidisciplinary interventions, optimized treatment, and health education.15 Indeed, the individuals included in our study were ambulatory patients who regularly attended a scheduled consult in a HF unit, in contrast to other published studies in which patients were enrolled immediately following hospital discharge.16,17,18 This difference in inclusion criteria and the dramatic reduction in hospitalizations found in this study might suggest that patients who are better treated and educated regarding their disease benefit the most from remote telemonitoring.
Current reports show that telemonitoring systems are associated with a trend toward improvement in morbidity, although the monitoring system which is best suited for each patient remains to be determined.9,19,20 In fact, discrepancies in the results obtained with different kinds of telemedicine is not surprising, given the differences in population, type of telemonitoring system used, and follow-up of the various studies.10,21 Some studies have obtained results contrary to ours with respect to reduced hospitalizations for HF. The TEN-HMS16 study, which compared a telemonitoring system with telephone support and usual care, showed that telemonitoring was associated with a tendency, though not significant, toward increased hospitalizations for HF (although with shorter in-hospital stays) when compared to monthly telephone support. This was explained in part by the early detection of decompensation facilitated by telemonitoring, which helped in the planning for hospital admissions and discharges, and the ease of use for this system concerning follow-up and control of ambulatory treatment.
A more recent study, HOME-HF,22 that implemented a telemonitoring program including self-monitoring equipment and questionnaires, did not exhibit a decrease in the time to first admission for patients in the telemonitoring arm compared with patients who followed usual care. On the other hand, the SHL,23 the Benatar et al.24, and SPAN-CHF25 studies obtained similar results to those presented in this study regarding the decrease in hospitalizations for HF, although in the latter 2 studies the follow-up period was only 3 months.
Although intended for patients with HF, our results were also positive regarding the decrease in admissions due to other cardiac causes; telemonitoring most likely allowed for early evaluation and treatment of symptoms through the bi-directional communication established between the patient and health care provider, either by telephone or through the information received (responses to the questionnaires and self monitoring). These results have also been obtained via methods such as telephone follow-up, with or without 24 h telephone assistance.17 Nevertheless, in other studies such as WHARF,18 one of the first studies to evaluate a remote disease management program in which a telemonitoring program (with self monitoring and questionnaires) was compared with care given in a HF program, a decrease in cardiac hospitalizations was not obtained.
Another aspect of our study that should be highlighted is the decrease in days in hospital for HF and for other cardiac causes. Such a reduction has also been observed in other studies that included self-monitoring equipment,23,24 and we believe it is due to the early detection and treatment of decompensation. This result is particularly relevant at present, when the availability of beds is a frequent limitation in public hospitals. It is remarkable that patients with self-monitoring equipment were admitted less often but required more visits to the unit, both with nurses and cardiologists. It is unclear whether these extra visits were directly related to the telemonitoring program.
A significant increase in patients’ perceived QoL was also found in our study. Here, QoL was assessed both with a specific HF questionnaire, the MLWHFQ, and a generic analogue scale, the EQ-VAS, after 1 year of follow-up. Other studies found a trend toward better QoL, although the trend did not reach statistical significance.18,22,24 In this study, the comparison of paired data allowed us to observe that a majority of patients perceived improvement in their QoL, especially in the physical dimension.
The number of voluntary de-installations in our study (22.7%) is worth noting, and differs from previous studies such as TEN-HMS,16 with only 7% in the telemonitoring arm, WHARF18 (11.4%), or HOME-HF,22 with only 1 of 84 patients withdrawing. Our belief is that the unwillingness to participate in this type of study is primarily a cultural or educational issue specific to our population cohort, perhaps still unfamiliar and uncomfortable with electronic systems.
In analyzing compliance in terms of responding to questionnaires and carrying out self-monitoring measures, we observed that the response rate for the questionnaires was high (89%) compared with self-monitoring of weight (76%) and blood pressure (71%). It is noteworthy that only 51.1% and 44.2% of patients controlled their weight and blood pressure, respectively, on 80% or more of the days of telemonitoring, in contrast to other studies in which the degree of compliance ranged from 80% in the HHH26 study or 81% in the TEN-HMS16 study (although only 55% achieved a compliance of twice daily, as instructed) and HOME-HF,22 in which 95% of patients utilized the system on more than 90% of the days. We did not find a plausible explanation for this worse compliance, beyond the longer duration of our study, and we can only hypothesize that, as in the case of patients who discontinued the study, there could be a cultural or educational component, or perhaps patients without a previous admission were less motivated. There is also no clear explanation for the fact that, despite the lower self-monitoring compliance, the decrease in hospital admissions was comparatively more pronounced in our study than in the other studies mentioned.
The main limitations of this study are those inherent to the before/after comparison design, and to the fact that these study patients attended a structured multidisciplinary HF unit, with treatment optimization and health education, making it impossible to extrapolate these results to other HF patients who were not so closely controlled. Moreover, patients treated in tertiary hospitals are usually selected patients, generally younger than the general population with HF. On the other hand, patients without TV or not being able to adequately carry out home self-monitoring were also excluded, which eventually could have altered the results.
ConclusionsThe Motiva telemonitoring system significantly reduced the number of hospitalizations and days in hospital for HF and other cardiac causes in patients controlled in a structured multidisciplinary HF unit. Although telemonitoring with self-monitoring equipment (scale and sphygmomanometer) showed an 83.9% reduction in days in hospital for HF whereas telemedicine without self-monitoring produced a 53.8% reduction, this difference did not reach statistical significance. In order to determine which patients might benefit more from a particular telemonitoring system, a larger sample, allowing for subgroup analysis, is required. Finally, patients significantly improved their perceived QoL, particularly in the physical dimension.
Conflicts of interestDr. Mar Domingo received a grant from Philips Inc. to develop the study and perform data collection. The other authors have no conflict of interest. Data analysis was carried out by the authors independently of the study sponsor.
FundingThe study was funded by Philips Healthcare and the Catalan Institute of Health.
Acknowledgments
The authors acknowledge Philips Healthcare, specifically Joan Valenzuela, for his collaboration and technical support, and Chris Westerteicher.
Received 5 August 2010
Accepted 30 October 2010
Corresponding author: Unitat d’Insuficiència Cardiaca, Hospital Universitari Germans Trias i Pujol, Ctra. de Canyet s/n, 08916 Badalona, Barcelona, Spain. jlupon.germanstrias@gencat.cat