Sodium-glucose cotransporter 2 inhibitors are a novel pharmacological class of oral hypoglycemic agents that lower glucose levels by increasing renal glucose excretion in an insulin-independent manner. However, this seemingly simple mechanism has more complex indirect metabolic effects. The results of randomized clinical trials have shown that these inhibitors effectively lower blood glucose and glycated hemoglobin levels without increasing the risk of hypoglycemia and, at the same time, also reduce bodyweight and systolic blood pressure. In this review, we describe the mechanism of action, efficacy, and safety of currently marketed drugs, as well as other risk factors besides glucose that can potentially be modulated positively. Recent data on empagliflozin showing a significant cardiovascular benefit have compelled us to update knowledge of this new therapeutic class for the treatment of type 2 diabetes.
Keywords
Type 2 diabetes mellitus (T2DM) is associated with elevated cardiovascular risk. Given the almost epidemic proportions of T2DM1, various management guidelines highlight the need to prevent and reduce cardiovascular complications by improving glycemic control, particularly in the early stages of the disease2. The overall therapeutic objective is to maintain blood glucose levels as close as possible to normal values in order to prevent or delay the onset of microvascular and macrovascular complications.
The number of drugs reducing or regulating glucose has increased in recent years, complicating and modifying the therapeutic approaches to T2DM. The current recommendations stress the individualization of glycemic targets. Combinations of various drugs with distinct mechanisms of action are typically required to achieve the recommended glycated hemoglobin (HbA1c) level of 7.0%, although the specific objective depends on the type of patient.
The recommended first-line treatment is metformin, followed by pioglitazone, a sulfonylurea, a dipeptidyl peptidase-4 inhibitor, a glucagon-like peptide-1 analog, or insulin on an individualized basis. Each drug has its own adverse effects and limitations, such as increased cardiovascular risk due to a direct effect of sulfonylureas on ischemic preconditioning3, weight gain, and hypoglycemia with insulin, and risk of heart failure with pioglitazone. The main drawback of incretin-based therapies is that a substantial proportion of patients will be nonresponders4. Thus, the development of novel therapies should focus on minimizing these phenomena while effectively and persistently reducing hyperglycemia.
The recent incorporation of a new therapeutic class of sodium-glucose cotransporter 2 (SGLT2) inhibitors provides an opportune moment for a conceptual review of T2DM therapies. These glucose-lowering agents reduce blood glucose levels, weight, and blood pressure by inducing glycosuria, a 3-pronged attack unique to oral hypoglycemic agents (OHAs). In addition, their mechanism of action does not depend on pancreatic beta cell function or insulin resistance and can indirectly improve both beta cell function and insulin action by reducing the glucotoxicity phenomenon.
The triple effect of this novel therapeutic class of drugs on risk factors, together with the unexpected benefits shown by empagliflozin on cardiovascular mortality, makes this group of drugs especially attractive for clinical cardiologists. In this review, we summarize the available data on this new class of therapeutics for T2DM.
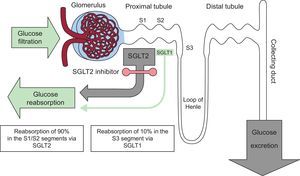
ROLE OF THE KIDNEY IN GLUCOSE METABOLISMIn addition to regulating glucose metabolism through glomerular filtration and glucose reabsorption in the proximal convoluted tubules, the kidney contributes to endogenous glucose release via gluconeogenesis. Although the liver is the main gluconeogenic organ during fasting, the renal contribution is still between 20% and 25%. In contrast, renal gluconeogenesis markedly increases in the postprandial period, becoming the source of 60% of endogenous glucose release and facilitating replenishment of hepatic glycogen reserves.
Between 160 and 180g of glucose is filtered by the kidneys every day in a healthy adult. Of this, more than 99% is reabsorbed from the proximal convoluted tubule to the peritubular capillaries via 2 proteins named sodium-glucose cotransporters (SGLTs): SGLT2 is expressed in the S1 and S2 anterior segments of the tubule, whereas SGLT1 is found in the S3 segment, but to a much greater extent in the enterocytes of the intestinal mucosa. The former is responsible for most reabsorption of the tubular glucose load (90%) via active transport (against a concentration gradient) within the proximal tubule. The remaining glucose (10%)5 is reabsorbed by SLGT1 in the most distal part of the tubule (Figure 1).
In T2DM, as an adaptive response to hyperglycemia, the expression and activity of SGLT2 are increased in epithelial cells of the tubule to minimize glycosuria, but this results in continuous glucose reabsorption, even in the presence of elevated plasma glucose concentrations6. Its endogenous release via renal gluconeogenesis is also tripled vs that of nondiabetic individuals7. Thus, renal processes, both reabsorption and production, represent a poor adaptation of T2DM patients that contributes to hyperglycemia and increases glucotoxicity. The possibility of intervening on these pathophysiological defects with SGLT2 inhibitors has led to reconsideration of the kidney as not only a victim of T2DM, but also an ally in its treatment.
RENAL SODIUM-GLUCOSE COTRANSPORTER 2 INHIBITORSThe following 3 inhibitors are currently available: dapagliflozin, empagliflozin, and canagliflozin.
DapagliflozinIn 2012, dapagliflozin became the first SGLT2 inhibitor to be approved in Europe. It is indicated for patients with inadequate control on metformin. Its selectivity for SGLT2 is 1200 times higher than that for SGLT1. The plasma concentration of dapagliflozin peaks at 1.5hours and it has a half-life of about 12hours8. The therapeutic dose of 10mg induces a mean glycosuria of 70g/day that results in a loss of 280kcal/day. This induced glycosuria is associated with higher muscle sensitivity to insulin and, paradoxically, with a higher glucagon concentration and endogenous glucose production, with little effect on glycemic control9. Its clinical development has been extensive, with more than 5000 randomized patients in 14 phase III clinical trials at different T2DM stages and with distinct treatments, both in monotherapy and in combination therapy with metformin, sulfonylureas, insulin, pioglitazone, and sitagliptin, and in specific populations such as patients with moderate renal failure and patients at high risk of cardiovascular events. Pooled analyses of the studies showed that dapagliflozin effectively reduces HbA1c by a mean of 0.79%10–14.
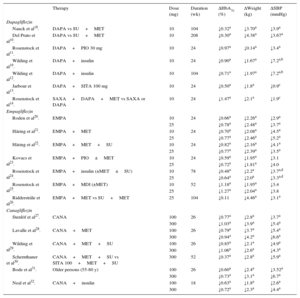
In monotherapy at 10mg/day, the inhibitor obtains a 0.89% reduction in HbA1c and weight loss of 3.2kg15. In combination with metformin, HbA1c is reduced by 0.84%, with a long-term weight loss of 2.86kg16,17.The main results from the pivotal studies are shown in Table 110–14,18–32.
Clinical Studies of Sodium-glucose Cotransporter 2 Inhibitors: Efficacy Results for Glycated Hemoglobin, Weight, and Systolic Blood Pressure
| Therapy | Dose (mg) | Duration (wk) | ΔHbA1c (%) | ΔWeight (kg) | ΔSBP (mmHg) | |
|---|---|---|---|---|---|---|
| Dapagliflozin | ||||||
| Nauck et al18. | DAPA vs SU+MET | 10 | 104 | ↓0.32a | ↓3.70a | ↓3.9a |
| Del Prato et al10. | DAPA vs SU+MET | 10 | 208 | ↓0.30a | ↓4.38a | ↓3.67a |
| Rosenstock et al11. | DAPA+PIO 30 mg | 10 | 24 | ↓0.97a | ↓0.14a | ↓3.4a |
| Wilding et al19. | DAPA+insulin | 10 | 24 | ↓0.90a | ↓1.67a | ↓7.2a,b |
| Wilding et al12. | DAPA+insulin | 10 | 104 | ↓0.71a | ↓1.97a | ↓7.2a,b |
| Jarbour et al13. | DAPA+SITA 100 mg | 10 | 24 | ↓0.50a | ↓1.8a | ↓0.9c |
| Rosenstock et al14. | SAXA+DAPA+MET vs SAXA or DAPA | 10 | 24 | ↓1.47a | ↓2.1a | ↓1.9c |
| Empagliflozin | ||||||
| Roden et al20. | EMPA | 10 | 24 | ↓0.66a | ↓2.26a | ↓2.9a |
| 25 | ↓0.78a | ↓2.48a | ↓3.7a | |||
| Häring et al21. | EMPA+MET | 10 | 24 | ↓0.70a | ↓2.08a | ↓4.5a |
| 25 | ↓0.77a | ↓2.46a | ↓5.2a | |||
| Häring et al22. | EMPA+MET+SU | 10 | 24 | ↓0.82a | ↓2.16a | ↓4.1a |
| 25 | ↓0.77a | ↓2.39a | ↓3.5a | |||
| Kovacs et al23. | EMPA+PIO±MET | 10 | 24 | ↓0.59a | ↓1.95a | ↓3.1 |
| 25 | ↓0.72a | ↓1.81a | ↓4.0 | |||
| Rosenstock et al24. | EMPA+insulin (±MET±SU) | 10 | 78 | ↓0.48a | ↓2.2a | ↓3.7a,d |
| 25 | ↓0.64a | ↓2.0a | ↓3.3a,d | |||
| Rosenstock et al25. | EMPA+MDI (±MET) | 10 | 52 | ↓1.18a | ↓1.95a | ↓3.4 |
| 25 | ↓1.27a | ↓2.04a | ↓3.8 | |||
| Ridderstråle et al26. | EMPA+MET vs SU+MET | 25 | 104 | ↓0.11 | ↓4.46a | ↓3.1a |
| Canagliflozin | ||||||
| Stenlöf et al27. | CANA | 100 | 26 | ↓0.77a | ↓2.8a | ↓3.7a |
| 300 | ↓1.03a | ↓3.9a | ↓5.4a | |||
| Lavalle et al28. | CANA+MET | 100 | 26 | ↓0.79a | ↓3.7a | ↓5.4a |
| 300 | ↓0.94a | ↓4.2a | ↓6.6a | |||
| Wilding et al29. | CANA+MET+SU | 100 | 26 | ↓0.85a | ↓2.1a | ↓4.9a |
| 300 | ↓1.06a | ↓2.6a | ↓4.3a | |||
| Schernthaner et al30. | CANA+MET+SU vs SITA 100+MET+SU | 300 | 52 | ↓0.37a | ↓2.8a | ↓5.9a |
| Bode et al31. | Older persons (55-80 y) | 100 | 26 | ↓0.60a | ↓2.4a | ↓3.52a |
| 300 | ↓0.73a | ↓3.1a | ↓6.7a | |||
| Neal et al32. | CANA+insulin | 100 | 18 | ↓0.63a | ↓1.8a | ↓2.6a |
| 300 | ↓0.72a | ↓2.3a | ↓4.4a | |||
Δ, mean variation vs baseline value; CANA, canagliflozin; DAPA, dapagliflozin; EMPA, empagliflozin; HbA1c, glycated hemoglobin; MDI, multiple daily injection of insulin (baseline bolus therapy); MET, metformin; PIO, pioglitazone; SAXA, saxagliptin; SBP, systolic blood pressure; SITA, sitagliptin; SU, sulfonylurea.
The SGLT2 inhibitor empagliflozin was approved for use in Europe in 2014. Its plasma concentration peaks at 1.5hours and it has a 12-hour half-life. It shows about 2500 times higher selectivity for SGLT2 vs SGLT1. At 10 and 25mg doses, it induces glycosuria of 66.4 and 78.4g/day, resulting in calorie losses of 265 and 313kcal/day, respectively. It also increases glucagon production33. The drug has been widely studied in 12 phase III clinical trials, with more than 14 000 patients in distinct T2DM stages and with diverse treatments, as well as in special populations such as patients in all renal failure stages and patients with cardiovascular events. In monotherapy or in combination, a 10-mg dose exerts a mean HbA1c reduction of about 0.73%, with a 0.82% reduction reported for a 25-mg dose, as well as weight loss of between 1% and 3.1% and systolic blood pressure (SBP) reduction20–25, even in patients with stage 2-3a renal failure34. The results of the main studies are shown in Table 120–26.
CanagliflozinAvailable in Europe since 2015, canagliflozin inhibitsboth SGLT2 and SGLT1, depending on the dose. The latter is the transporter in charge of glucose and galactose absorption in the gastrointestinal tract. The plasma concentration of the drug peaks after 1 to 2hours and it has a 13-hour half-life. Its selectivity is 160 times higher for SGLT2 than for SGLT1. At a 300-mg dose, it has a clinically important effect on intestinal SGLT1, possibly due to a high intraluminal concentration of the drug before its absorption. One possible advantage of the dual inhibition is reduced intestinal glucose absorption, which could stimulate glucagon-like peptide 1 release. This peptide would then enhance postprandial insulin secretion and help to improve postprandial blood glucose levels. Doses of 100 and 300mg induce mean glycosuria of 70 and 119g/day and promote the loss of 308 and 476kcal/day, respectively35. Its clinical development has been extensive, with 15 phase III trials and more than 10 285 patients in different stages and with different treatments of T2DM and in special populations such as patients older than 75 years, those with moderate renal failure, and those with a history of cardiovascular events. The studies27–32 (Table 1) have shown the ability of the drug to reduce HbA1c in monotherapy and in dual or triple therapy with OHAs or combined with insulin. With 100 and 300mg, the mean HbA1c reductions are 0.72% and 0.89%, respectively27–29,31,32. When canagliflozin 300mg is compared with sitagliptin 100mg combined with metformin and sulfonylurea, a greater HbA1c reduction is achieved than with sitagliptin (0.37%), as well as a weight loss of 2.8kg30. All clinical studies of canagliflozin have shown persistent 1.0% to 3.8% reductions in body weight and SBP27–32.
In patients with T2DM and renal failure, SGLT2 inhibitors show reduced efficacy due to their mechanism of action, although they have a good safety profile34,36,37. This type of patient can show a temporary deterioration in glomerular filtration due to hemodynamic factors with no signs of renal damage, as indicated by the albumin/creatinine ratio. Precaution is recommended with all drugs of this class when a blood pressure fall could be a risk, for example, when patients are on antihypertensive therapy and have a history of hypotensive episodes or are older than 75 years. The indications for SGLT2 inhibitor initiation and dose adjustment according to the glomerular filtration rate and the age established in the technical data sheets are shown in Table 2.
Indications for Sodium-glucose Cotransporter 2 Inhibitor Initiation and Dose Adjustment Based on Glomerular Filtration Rate and Age
| eGFR ≥ 60 mL/min/1.73 m2 | eGFR ≥ 45-60 mL/min/1.73 m2 | eGFR ≥ 30-45 mL/min/1.73 m2 | eGFR<30 mL/min/1.73 m2 | Age | |
|---|---|---|---|---|---|
| Dapagliflozin | • Start with 10 mg/daya | • Do not start • Discontinue if receiving treatment and GFR<60b | • Do not start • Discontinue if receiving treatment | • Do not start • Discontinue if receiving treatment | <75 y |
| Empagliflozin | • Start with 10 mg/day • Increase to 25 mg/day if there is good tolerance and need for better glycemic control | • Start with 10 mg/day* • Increase to 25 mg/day if there is good tolerance and need for better glycemic control | • Do not start • Discontinue if receiving treatment and GFR<45b | • Do not start • Discontinue all drug doses | <85 yc |
| Canagliflozind | • Start with 100 mg/day • Increase to 300 mg/day if there is good tolerance and need for better glycemic control | • Start with 100 mg/day and do not increase** • Reduce to 100 mg/day if patient is being treated with 300 mg | • Do not start • Discontinue if receiving treatment and GFR<45b | • Do not start • Discontinue all drug doses | >18 ye |
eGFR, estimated glomerular filtration rate; GFR, glomerular filtration rate.
Although its pharmacokinetics are not affected by food, it should be taken before the first food intake of the day due to its potential ability to delay intestinal absorption of glucose.
In addition to glucose reduction and improved insulin sensitivity, these drugs have favorable effects on classic cardiovascular risk factors, such as blood pressure and weight, and also modify other parameters such as visceral adiposity, arterial stiffness, albuminuria, and uric acid concentration. However, it is still unclear whether these effects reduce cardiovascular events.
Blood Pressure and Arterial StiffnessAll studies of SGLT2 inhibitors have found significant reductions in blood pressure, which are higher for systolic (from 1.66 to 6.9mmHg) than diastolic (from 0.88 to 3.5mmHg) pressure. The initial reduction in SBP is believed to be due to osmotic diuresis effects induced by glycosuria, natriuresis, and reduced intravascular volume and is not accompanied by a heart rate increase, a finding that has been interpreted as indicating a relative reduction in the sympathetic nervous system tone38. However, their long-term effects could be due to inhibition of the renin-angiotensin-aldosterone system (RAAS) and weight loss.
In a pooled analysis of 6 phase III studies of more than 4000 patients treated with canagliflozin, there were moderate reductions in SBP vs placebo (3.3 and 4.5 mmHg with 100 and 300 mg, respectively)39. Similarly, the data of 4 phase III trials with more than 2000 patients treated with empagliflozin (10 or 25mg), both in monotherapy and in combination, revealed significant SBP reductions in the treatment group40. The duration of the effect is controversial because the SBP reductions were temporary in a 2-year study of dapagliflozin, with the pressure values returning to baseline levels12. Although the reduction is moderate, a SBP decrease of 5mmHg is nonetheless associated with a relative risk reduction of serious cardiovascular events of 14.2%41.
These inhibitors can help to improve the vascular architecture by modulating the connective tissue components involved in the development of arterial stiffness42. Indeed, empagliflozin has been shown to directly reduce arterial stiffness43.
Body Weight and Fat MassMost patients treated with SGLT2 inhibitors lose weight. This effect should be beneficial, given the potential association of obesity with vascular events in T2DM44. The initial weight loss is probably due to a diuretic effect and intravascular volume reduction induced by the drugs. However, the long-term weight loss could be due to calorie loss caused by glycosuria (265-476 kcal/day).
Dapagliflozin obtains mean body weight reductions of 3kg the first year, which are sustained at 2 years12. Canagliflozin has a similar effect on weight, with reductions of between 1.0% and 3.8% after 6 months of treatment27–32. In a longer-term study, Rosenstock et al24. reported a sustained weight loss of 2.2kg in patients treated with empagliflozin and basal insulin24.
Although the weight losses could be considered moderate, studies have shown that a loss of>2.25 kg induces a decrease in the cardiovascular risk factor sum of 48% in men and 40% in women after 16 years of follow-up45.
Of potentially greater interest is the change in visceral fat, given its greater association with risk of cardiovascular complications. The weight loss is mainly due to reduced visceral fat, as shown by body composition studies of SGLT2 inhibitors26,46,47. Reductions in indirect markers of visceral adiposity were also seen in short-term studies48. One ongoing study is examining the influence of dapagliflozin on epicardial adipose tissue with the hypothesis that its reduction would improve cardiac contractility49.
Proteinuria and Renal FunctionIn patients with T2DM, the elevated SGLT2 expression increases sodium reabsorption, decreasing solute delivery to the macula densa and leading to afferent arteriolar vasodilation and renal hyperfiltration. These initial hemodynamic and tubular changes promote the onset and progression of microalbuminuria and a gradual decrease in the glomerular filtration rate. Inhibition of SGLT2 has both direct and indirect nephroprotective effects: direct via neutralization of this defect by inducing natriuresis, which causes afferent arteriolar vasoconstriction and intraglomerular pressure reduction with decreased hyperfiltration50, and indirect via restriction of glucose entry into the peritubular cells, which improves the levels of inflammatory and fibrotic markers, as shown with empagliflozin51. Specific renal studies of SGLT2 inhibitors found regression and reduction of microalbuminuria and decreased macroalbuminuria34,37,52. These effects on renal function cause a proteinuria decrease of between 30% and 40% that is independent of the SBP, HbA1c, and weight reductions. In addition, the nonclassic RAAS pathway can influence renal protection through angiotensin-converting enzyme, which degrades angiotensin II to angiotensin 1/7 with consequent vasodilatory, anti-inflammatory, and antiproliferative effects. The use of SGLT2 inhibitors in patients with T2DM treated with RAAS inhibitors can have beneficial effects on diabetic nephropathy by activating the nonclassic RAAS pathway50.
Effects on the Lipid ProfileSome studies have found minor changes in lipid levels. In one of these studies, dapagliflozin in combination with metformin increased the high-density lipoprotein-cholesterol (HDL-C) level (from 1.8% to 4.4% vs baseline) and reduced the triglyceride level (from 2.4% to 6.2% vs baseline) compared with placebo after 6 months of treatment16. Canagliflozin increased both HDL-C (7.6%) and low-density lipoprotein-cholesterol (LDL-C) (11.7%)47.
In 2-year studies of dapagliflozin, empagliflozin, and canagliflozin, the LDL-C increases were 5, 6, and 3mg/dL and the HDL-C increases were 1.0, 3.5, and 0.6mg/dL, respectively, with a mean triglyceride decrease of 9 to 10mg/dL53. A meta-analysis of clinical studies performed with the different drugs showed a moderate but significant increase in HDL-C values without changes in LDL-C and triglyceride levels54. More studies are required to determine the clinical relevance of these findings.
Effects on Uric AcidIn addition to decreasing the blood glucose level, these inhibitors have been shown to reduce the serum uric acid concentration. A post hoc analysis of the data from various clinical studies of canagliflozin showed a uric acid decrease of 13% (0.7 mg/dL)55. In addition, in the cohort with hyperuricemia, the proportion of patients achieving serum uric acid concentrations<6 mg/dL at week 26 was 23.5% with canagliflozin 100mg and 32.4% with 300mg vs 3.1% with placebo. In patients with baseline hyperuricemia, there was a similar incidence of gout and kidney stones, without differences vs placebo55. Similar data have been reported for dapagliflozin17 and empagliflozin20,22,23.
This reduction in uric acid titers is possibly related to GLUT9, a transporter that passively excretes urate into the urine and exchanges it for glucose. It remains to be seen if this effect can be converted into long-term beneficial results, whether on renal function or on macrovascular complications.
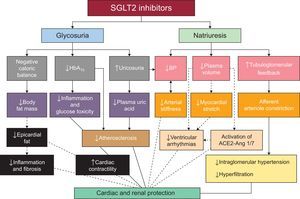
The possible pathophysiological mechanisms underlying the cardiovascular and renal protective effects of the SGLT2 inhibitors are illustrated in Figure 256.
Possible mechanisms of cardiovascular and renal protection of sodium-glucose cotransporters 2. The continuous lines show mechanisms demonstrated by existing data. The broken lines indicate other possible mechanisms under study. ACE, angiotensin-converting enzyme; Ang 1/7, angiotensin 1/7; BP, blood pressure; HbA1c, glycated hemoglobin; SGLT2, sodium-glucose cotransporter 2. Adapted with permission from Rajasekeran et al56.
Hypoglycemia hinders the achievement of control targets, especially in patients with cardiovascular disease, due to its association with ventricular arrhythmias. Thus, physicians must always remember the hypoglycemia-macroangiopathy tandem when planning the therapeutic strategy.
Because their mechanism of action is independent of that of insulin, SGLT2 inhibitors produce very low rates of hypoglycemia (0.9%-4.3%), which additionally tends to be mild. The greatest benefits are seen when the inhibitors are combined with sulfonylureas or insulin. Thus, the dosages of both drugs should be reduced when they are combined with SGLT2 inhibitors to minimize the risk of hypoglycemia57.
Genitourinary InfectionsAs these drugs induce glycosuria, they can facilitate the development of genital and urinary tract infections. A systematic review of the safety data of patients treated with dapagliflozin58 showed a clear association between the drug and genital mycotic infections (5% in treated patients vs 0.9% in the placebo group). These infections were generally mild and responded to standard antifungal therapy. The first episode usually occurs during the first months of treatment in both sexes and can be recurrent in a minority. The most frequent diagnoses are mycotic balanitis and vulvovaginitis and the infection occurrence rate is 7 times higher in women, particularly those who are premenopausal with history of genital infections and obesity; the infections are independent of the HbA1c level.
Urinary tract infections, common in patients with T2DM, are not significantly increased by the use of SGLT2 inhibitors. Studies of dapagliflozin 10mg/day showed a minor increase in infections vs placebo (4.3% vs 3.7%)59. The symptoms were typical, responded to conventional antibiotics, and did not prompt therapy discontinuation. The main predisposing factors were age>65 years, female sex, and history of recurrent infections. Similar data have been reported for empagliflozin60 and canagliflozin30. With the latter drug, the infection incidence was somewhat increased in elderly patients and those with T2DM for more than 12 years.
KetoacidosisTreatment with SGLT2 inhibitors can increase the risk of the development of a type of ketoacidosis called euglycemic diabetic ketoacidosis61, with most cases occurring in patients with type 1 diabetes mellitus. This ketoacidosis has also been reported in some T2DM patients, particularly patients with diminished pancreatic reserve due to long-term disease or with latent pancreatic autoimmunity or those who belong to ethnic groups whose T2DM has a rapid tendency to ketosis. The prime characteristic of this type of ketoacidosis is that decompensation is often missed because patients have blood glucose levels<250mg/dL due to glycosuria and only moderately elevated ketonuria, findings that do not suggest that the symptoms are secondary to ketoacidosis and that thereby delay the corrective intervention. Precipitating factors can be identified in some patients, such as insulin reduction or suppression, intercurrent conditions, reduced nutritional intake due to acute disease or surgery, and alcohol abuse. This condition is an approved indicator for only T2DM and cannot be used as an indicator of type 1 diabetes mellitus until a new indication is approved, pending the results of ongoing clinical trials. Use of the inhibitors should be temporarily discontinued to prevent situations that can cause dehydration or prolonged fasting and for elective surgery.
Cardiovascular SafetyAlthough SGLT2 inhibitors have beneficial effects on classic cardiovascular risk factors, specific studies have had to be performed to evaluate their effects on cardiovascular risk, in accordance with the rules of international regulatory bodies.
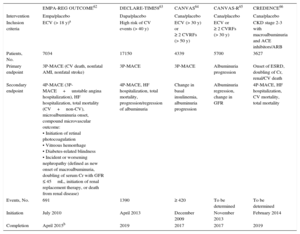
These studies have a number of differences in both the numbers and types of diabetic patients included (eg, patients with established cardiovascular disease or risk factors for its development, or a combination of both). A characteristic shared by 3 of them62–64 is the primary endpoint, a composite outcome of cardiovascular death, acute myocardial infarction (AMI), and nonfatal stroke, whereas the other 2 were mainly focused on renal outcomes (Table 3)65,66.
Design Differences Among Studies of Cardiovascular Safety With Sodium-glucose Cotransporter 2 Inhibitors
| EMPA-REG OUTCOME62 | DECLARE-TIMI5863 | CANVAS64 | CANVAS-R65 | CREDENCE66 | |
|---|---|---|---|---|---|
| Intervention | Empa/placebo | Dapa/placebo | Cana/placebo | Cana/placebo | Cana/placebo |
| Inclusion criteria | ECV (> 18 y)a | High risk of CV events (> 40 y) | ECV (> 30 y) or ≥ 2 CVRFs (> 50 y) | ECV or ≥ 2 CVRFs (> 30 y) | CKD stage 2-3 with macroalbuminuria and ACE inhibitors/ARB |
| Patients, No. | 7034 | 17150 | 4339 | 5700 | 3627 |
| Primary endpoint | 3P-MACE (CV death, nonfatal AMI, nonfatal stroke) | 3P-MACE | 3P-MACE | Albuminuria progression | Onset of ESRD, doubling of Cr, renal/CV death |
| Secondary endpoint | 4P-MACE (3P-MACE+unstable angina hospitalization), HF hospitalization, total mortality (CV+non-CV), microalbuminuria onset, compound microvascular outcome: • Initiation of retinal photocoagulation • Vitreous hemorrhage • Diabetes-related blindness • Incident or worsening nephropathy (defined as new onset of macroalbuminuria, doubling of serum Cr with GFR ≤ 45mL, initiation of renal replacement therapy, or death from renal disease) | 4P-MACE, HF hospitalization, total mortality, progression/regression of albuminuria | Change in basal insulinemia, albuminuria progression | Albuminuria regression, change in GFR | 4P-MACE, HF hospitalization, CV mortality, total mortality |
| Events, No. | 691 | 1390 | ≥ 420 | To be determined | To be determined |
| Initiation | July 2010 | April 2013 | December 2009 | November 2013 | February 2014 |
| Completion | April 2015b | 2019 | 2017 | 2017 | 2019 |
3P-MACE, composite variable of 3 major adverse cardiovascular events; 4P-MACE, composite variable of 4 major adverse cardiovascular events; ACE, angiotensin-converting enzyme; AMI, acute myocardial infarction; ARB, angiotensin receptor blockers; Cana, canagliflozin; CKD, chronic kidney disease; Cr, serum creatinine; CV, cardiovascular; CVRFs, cardiovascular risk factors; Dapa, dapagliflozin; ECVD, established cardiovascular disease; Empa, empagliflozin; ESRD, end-stage chronic renal disease; GFR, glomerular filtration rate; HF, heart failure; MACE, major adverse cardiovascular events.
The EMPA-REG OUTCOME study62 was designed to examine the long-term effects of the addition of empagliflozin to standard care on the morbidity and mortality of 7020 patients with T2DM for more than 10 years and established cardiovascular disease. The results show a clear and rapid reduction in cardiovascular mortality, not wholly explainable by the 0.4% decrease in the HbA1c level, 5mmHg lower SBP, or 3% weight loss. In the classic studies of the effects of interventions on cardiovascular risk factors, many years of follow-up were required to detect a mortality reduction.
The primary endpoint was a composite outcome of cardiovascular death, AMI, and nonfatal stroke. Endpoint incidence was significantly reduced by 14% in the empagliflozin group, with decreases in cardiovascular mortality of 38% and heart failure admissions of 35%, without differences in the rates of AMI and nonfatal stroke.
Among the mechanisms possibly underlying the clinical benefit observed, the most likely mechanism is the diuretic effect. The rapid reduction (within 2-4 months) in cardiovascular events in the empagliflozin-treated group suggests a hemodynamic effect. The SBP decrease was highly significant at 1 month and peaked at 4 months (∼5 mmHg), the same as the weight loss. In addition, the intravascular volume decrease persisted until the end of the study, as indicated by the sustained hematocrit increase (4.8%). This temporal association could explain the reduction in cardiovascular events in the empagliflozin-treated group in the initial months.
The mortality reduction was much more pronounced in diabetic patients without heart failure, which is why the other proposed mechanisms are independent of the diuretic effect. These other mechanisms include activation of the nonclassic RAAS pathway by the SBP and volume decrease, which would trigger the production of angiotensin 1/7 with cardioprotective effects in patients treated with angiotensin-converting enzyme antagonists or angiotensin II receptor antagonists (81%)67.
Another factor to consider would be that these drugs increase the glucagon concentration, whose positive inotropic and antiarrhythmic effects could at least partly explain the improved cardiovascular mortality and heart failure hospitalization rates68.
Empagliflozin also decreases arterial stiffness, cardiac afterload43, indirect markers of visceral adiposity48, and microalbuminuria34. However, because their clinical benefits are delayed, they do not seem to be essential for short-term event reduction.
In addition, the EMPA-REG OUTCOME trial found favorable effects on microvascular events included in the secondary endpoints (Table 3)69, which were significantly reduced by 39% in the empagliflozin-treated group. The magnitude of this decrease had a strong effect on nephropathy, with relative risk reductions of 39% in incident or worsening nephropathy, 38% in progression to macroalbuminuria, 44% in doubling of the serum creatinine level with a decreased glomerular filtration rate ≤ 45mL/min/1.73 m2, and 55% in the need for renal replacement therapy. As with the cardiovascular results, the benefits were evident in the first 3 months and were maintained until the end of the study. When the patients were categorized according to the glomerular filtration rate, the beneficial effect on heart failure hospitalization was in line with previously reported data, with a 41% reduction in those with a glomerular filtration rate<60mL/min and a 30% reduction in those with a glomerular filtration rate ≥ 60mL/min.
These results were seen in a patient population largely treated with RAAS inhibitors—the drugs recommended for diabetic nephropathy—supporting the potential combination use of empagliflozin and RAAS inhibitors in patients with T2DM and chronic renal disease.
From a metabolic point of view, a partial explanation of the study results—with the cardiovascular and renal benefits rapidly evident without a reduction in traditional atherothrombotic events—could be the mild but persistent ketosis caused by SGLT2 inhibitor therapy. Beta-hydroxybutyrate may be taken up in the heart and oxidized in place of fatty acids and glucose, constituting a more efficient source of myocardial energy. This substrate selection would improve the myocardial efficiency of oxygen consumption and could improve the metabolic state and function of other organs, particularly the kidneys70.
The EMPA-REG OUTCOME study is the first to clearly show that an OHA reduces cardiovascular events in patients with T2DM and established cardiovascular disease, as well as clinically important renal events, because it shows slower renal disease progression and also confirms the safety profile of SGLT2 inhibitors. Although these results are encouraging and have important clinical implications, it is necessary to consider whether these benefits are applicable to other T2DM patient profiles and whether they represent a class effect of the inhibitors.
DapagliflozinIn a 20-year simulation study, the addition of dapagliflozin to available therapeutic options resulted in relative reductions in the incidence of AMI, stroke, and cardiovascular death of 13.8%, 9.1%, and 9.6%, respectively71. The results of a meta-analysis of 21 phase II/III studies characterizing the cardiovascular profile of dapagliflozin that pooled patients according to risk (cardiovascular disease, number of risk factors, and age) did not find an increased risk, indicating a possible beneficial effect in all patient subgroups72.
Although these findings appear to indicate no association between dapagliflozin therapy and cardiovascular risk, no definite conclusion can be made until the completion of the DECLARE-TIMI58 study63 in 2019.
CanagliflozinIn the CANVAS study64, whose intermediate results were used for canagliflozin approval, there was no cardiovascular damage in the first 18 months of treatment, only a nonsignificant increase in fatal and nonfatal stroke73. This study, begun in 2009, will continue until the appearance of a minimum of 420 events of the primary endpoint, possibly limiting its potential use in the evaluation of cardiovascular benefits.
CONCLUSIONSThe new SGLT2 inhibitors are effective in the short- and medium-term for the management of hyperglycemia in T2DM patients, both in monotherapy and in combination with any of the available OHAs and insulin. In addition, beneficial effects beyond glycemic control are seen, such as weight and SBP reductions. These drugs have a good safety profile and one of them, empagliflozin, even achieves a reduction in cardiovascular mortality in diabetic patients with established cardiovascular disease. The results of ongoing studies are vital to determine whether the findings can be extended to the entire therapeutic group. Currently, the available data provide a solid basis for the use of empagliflozin in patients with T2DM and established cardiovascular disease.
Accordingly, we expect that SGLT2 inhibitors will soon occupy a prominent position in the therapeutic algorithms for T2DM.
CONFLICTS OF INTERESTThe authors have participated as researchers in the EMPA-REG OUTCOME study.