The management and risk stratification of patients with atrial fibrillation (AF) and acute coronary syndromes constitute a challenge. We aimed to evaluate the prognostic impact of AF whether present at admission or occurring during hospitalization for acute coronary syndromes, as well as trends in treatments and outcome.
MethodsData derived from 35 958 patients enrolled between 2004 and 2015 in the AMIS Plus registry were retrospectively analyzed.
ResultsPre-existing AF (pre-AF) was present in 1644 (4.7%) while new-onset AF (new-AF) was evident in 309 (0.8%). Presentation with ST-segment elevation myocardial infarction and need for hemodynamic support was frequent in patients with AF, especially in those with new onset of the arrhythmia. A change of the medical and interventional approaches was observed with a progressive increase in oral anticoagulation prescription and referral for angiography and percutaneous coronary interventions in pre-AF patients. Despite different baseline risk profile and clinical presentations, both AF groups showed high in-hospital and 1-year mortality (in-hospital new-AF vs pre-AF [OR, 0.79; 95%CI, 0.53-1.17; P = .246]; 1-year mortality new-AF vs pre-AF [OR, 0.72; 95%CI, 0.31-1.67; P = .448]) Pre-AF but not new-AF independently predicted in-hospital mortality. While mortality declined over the study period for patients with pre-AF, it remained stable among new-AF patients.
ConclusionsWhile pre-AF is independently associated with in-hospital mortality, new-AF may reflect a worse hemodynamic impact of the acute coronary syndromes, with the latter ultimately driving the prognosis.
Keywords
The management of patients with atrial fibrillation (AF) and acute coronary syndrome (ACS) constitutes a challenge due to the paucity of data on the patient's specific bleeding and embolic risks and on appropriate antithrombotic treatment.1 In the absence of dedicated randomized controlled trials, the available evidence is mainly derived from subgroup analyses of stent or AF trials as well as registries. Overall, in the setting of ACS, AF patients appear to have worse prognosis and less access to invasive treatments compared with non-AF patients.2–11 However, it is still a matter of debate whether AF adversely affects prognosis in ACS per se or whether it is a marker of comorbidities, the latter effectively driving the outcome.2,5,6,8,9,11
While in most cases, AF is chronic and unrelated to ACS, on occasion patients develop AF in the acute setting. The pathophysiological mechanisms, the relative clinical impact on short and long-term outcomes and the management of AF in these 2 circumstances differ substantially.5,6,8–11 Moreover, whether the occurrence of AF during an ACS represents a predictor of in-hospital mortality or a marker of hemodynamic instability, the latter ultimately driving the prognosis, is still unknown.
The aim of this study was to evaluate the differential prognostic impact of AF, whether present at admission or occurring during hospitalization for ACS, as well as trends in treatments and outcomes according to the time of onset of AF.
METHODSPatient PopulationAcute Myocardial Infarction in Switzerland (AMIS) Plus is a nationwide prospective registry enrolling patients admitted with ACS with or without ST-segment elevation in more than 80 hospitals recruiting since 1997.12,13 Patients are enrolled on a voluntary basis by signing a written informed consent form. Participating centers provide data for each patient through a standardized questionnaire including 200 items.
Data collection is centralized at the Institute of Social and Preventive Medicine of the University of Zürich. All data are checked for completeness, plausibility, and consistency by the AMIS Plus Data Center, querying treating physicians if the data are incomplete. Since 2010, external monitoring is regularly performed in randomly selected hospitals. The registry was approved by the Swiss Federal Ethics Committee for Clinical Studies, the Swiss Board for Data Security, and the appropriate Cantonal Ethics Commissions. The study protocol adheres to the ethics guidelines of the Declaration of Helsinki.
In this analysis, patients enrolled between January 1st, 2004 to July 1st, 2015 were considered. For all patients, detailed clinical history, in-hospital medical/interventional treatments and complications, therapy and vital status at discharge were available. Data from patients evaluated with coronary angiography are also reported. The Charlson comorbidity index, a quantitative estimate of associated comorbidities, was calculated.14,15
Among all in-hospital complications collected in the registry, those considered in the present analysis were defined as follows: a) cardiogenic shock: persistent hypotension (systolic blood pressure < 90mmHg) with clinical signs of severe reduction in cardiac index; b) stroke or transient ischemic attack: as any event due to ischemic, thrombotic or hemorrhagic disturbances confirmed by a neurologist or imaging modality; c) reinfarction: clinical signs or symptoms of ischemia with electrocardiogram changes indicative of new ischemia (new ST-changes or new left bundle branch block) and a new rise of biomarkers following the initial infarction; d) bleedings: recorded if deemed clinically relevant by the individual physician caring for the patient, without the use of a classification system.
At the time of the initial hospitalization, patients included in the registry may consent on an individual basis to a 1-year follow-up by telephone interview. Therefore the 1-year follow-up reported here reflects events occurring between the time of hospital discharge and 1 year only in the subgroup of patients accepting a telephone follow-up visit.
To better evaluate the clinical and prognostic impact of different timing of onset of AF in ACS, we classified patients on the basis of the time when the arrhythmia became clinically evident. Patients with AF documented at admission were classified as patients with pre-existing AF (pre-AF). This group therefore included patients with permanent or persistent AF and those who developed AF before hospital admission. Patients showing sinus rhythm at admission and developing AF at any time during hospitalization persisting until discharge, were considered as those with episodes of new-onset AF (new-AF).
Statistical AnalysisData are presented as the proportion of valid cases for discrete variables and as mean ± standard deviation and/or medians with interquartile ranges for continuous variables. Differences in baseline characteristics were compared using the unpaired t test or Mann-Whitney U test, if appropriate, and the Pearson chi-square test. Bonferroni correction for multiple comparisons was adopted. Statistics for each table are based on all cases with valid data in the specified ranges for all variables in each table.
Univariate and multivariate analyses were performed using logistic regression analysis with a stepwise approach to identify predictors of in-hospital mortality in the whole population. The results of logistic regression are reported as odds ratios (OR) with a 95% confidence interval (95%CI). For the trend analysis, the Mantel Haenszel linear by linear association chi-square test with 1 degree of freedom was used. A probability value of P < .05 was considered significant. The IBM SPSS Statistics Version 22 (Armonk, New York: IBM Corp) was used for other statistical analyses.
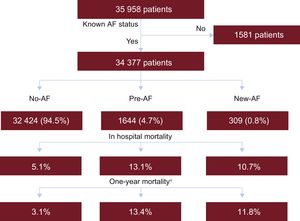
RESULTSPatient CharacteristicsThe AMIS Plus registry included 35 958 patients admitted for ACS in the predefined study period, and data on heart rhythm at admission were available for 34 377. Of them, 1644 (4.7%) had AF at admission. In 309 (0.8%) patients, initially admitted with stable sinus rhythm, episodes of AF occurred during the hospital stay. Figure 1 reports patient flow according to group allocation with in-hospital and 1-year mortality, while Table 1 reports baseline characteristics.
Patient flow according to group allocation with in-hospital and 1-year mortality. AF, atrial fibrillation; No-AF: patients without atrial fibrillation; New-AF: patients with new-onset atrial fibrillation; Pre-AF: patients with pre-existing atrial fibrillation. *Subgroups of patients followed up to 1-year. A total of 8534 had no AF, 357 had AF at admission and 68 had AF during hospitalization.
Baseline Clinical Characteristics
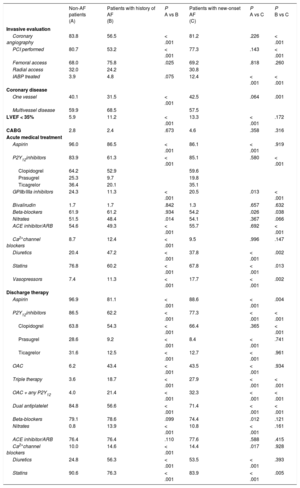
| Non-AF patients (A) | Patients with history of AF (B) | P A vs B | Patients with new-onset AF (C) | P A vs C | P B vs C | |
|---|---|---|---|---|---|---|
| Number of patients | 32 424 | 1644 | 309 | |||
| Female sex, % | 26.3 | 34.0 | < .001 | 31.4 | .02 | .180 |
| Age, y | 65.6 ± 13.2 | 76.8 ± 10.7 | < .001 | 74.6 ± 10.9 | < .001 | .001 |
| Risk factors | ||||||
| Family history, % | 34.0 | 26.6 | < .001 | 24.4 | .001 | .481 |
| Smoking, % | 39.4 | 22.6 | < .001 | 23.5 | < .001 | .739 |
| Dyslipidemia, % | 58.2 | 53.5 | .006 | 52.0 | .041 | .659 |
| Hypertension, % | 61.6 | 79.3 | < .001 | 68.4 | .010 | < .001 |
| Diabetes, % | 20.0 | 29.0 | < .001 | 26.1 | .009 | .324 |
| BMI > 30, % | 21.3 | 21.4 | .964 | 24.1 | .284 | .336 |
| Comorbidities | ||||||
| CAD, % | 33.9 | 42.8 | < .001 | 24.6 | .799 | < .001 |
| Heart failure, % | 2.6 | 10.4 | < .001 | 5.4 | .003 | .007 |
| CVD, % | 5.4 | 11.4 | < .001 | 8.8 | .010 | .181 |
| PVD, % | 5.1 | 10.0 | < .001 | 8.8 | .002 | .623 |
| Renal impairment (KDOQI class > 3), % | 6.6 | 17.2 | < .001 | 9.1 | < .001 | .041 |
| CHADS2 score, median | 1 [0-2] | 2 [1-3] | 2 [1-3] | |||
| CHA2DS2-VASC score, median | 2 [1-3] | 3 [2-4] | 3 [2-4] | |||
| Presentation | ||||||
| STEMI, % | 55.3 | 47.5 | < .001 | 61.5 | .028 | < .001 |
| Out of hospital CA, % | 4.8 | 7.8 | < .001 | 9.1 | < .001 | .447 |
| Killip class 3 or 4, % | 6.5 | 15.7 | < .001 | 13.4 | < .001 | .319 |
| Chest pain, % | 87.6 | 75.4 | < .001 | 85.2 | .209 | < .001 |
| Dyspnea, % | 29.6 | 49.6 | < .001 | 44.0 | < .001 | .092 |
AF, atrial fibrillation; BMI, body mass index; CA, cardiac arrest; CAD, coronary artery disease; CVD, cerebrovascular disease; KDOQI, Kidney Disease Outcomes Quality Initiative; PVD, peripheral vascular disease; STEMI, ST-segment elevation myocardial infarction.
Data are expressed as No. (%), mean ± standard deviation or median [interquartile range].
Patients with pre-AF were older and showed a greater burden of associated comorbidities such as hypertension, diabetes, known coronary/peripheral artery disease, heart failure and cerebrovascular disease than those without the arrhythmia. Of note, acute admissions for ST-segment elevation myocardial infarction (STEMI) or typical chest pain were less frequent, while more severe presentations such as cardiogenic shock with pulmonary overload and out of hospital cardiac arrest were more frequent in pre-AF than in non-AF patients.
Patients with new-AF showed some distinguishing peculiarities compared with patients without AF as well as those with pre-AF. In fact, while being older than non-AF patients, they were significantly younger than patients with known AF and had fewer comorbidities. New-AF patients had the highest rates among the 3 groups of STEMI presentation (61.5%), as well as out of hospital cardiac arrest (9.1%).
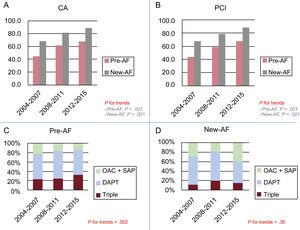
Acute ManagementTable 2 reports the in-hospital acute treatments and discharge therapy of patients according to their allocation group. Pre-AF patients less frequently received invasive diagnostics and percutaneous coronary interventions (PCI) than patients without known AF (angiograms pre-AF 56.5% vs non-AF 83.8%, P < .001; PCI pre-AF 53.2% vs non-AF 80.7%, P < .001; Table 2). In pre-AF patients, a trend toward increased referrals for coronary angiography and PCI was evident between 2004 and 2015 with an approximate 2 fold rise in the number of PCI (Figure 2A and B). When angiograms were performed, transfemoral access was chosen in 3 out of 4 patients.
In-hospital Acute Treatments and Discharge Therapies
| Non-AF patients (A) | Patients with history of AF (B) | P A vs B | Patients with new-onset AF (C) | P A vs C | P B vs C | |
|---|---|---|---|---|---|---|
| Invasive evaluation | ||||||
| Coronary angiography | 83.8 | 56.5 | < .001 | 81.2 | .226 | < .001 |
| PCI performed | 80.7 | 53.2 | < .001 | 77.3 | .143 | < .001 |
| Femoral access | 68.0 | 75.8 | .025 | 69.2 | .818 | .260 |
| Radial access | 32.0 | 24.2 | 30.8 | |||
| IABP treated | 3.9 | 4.8 | .075 | 12.4 | < .001 | < .001 |
| Coronary disease | ||||||
| One vessel | 40.1 | 31.5 | < .001 | 42.5 | .064 | .001 |
| Multivessel disease | 59.9 | 68.5 | 57.5 | |||
| LVEF < 35% | 5.9 | 11.2 | < .001 | 13.3 | < .001 | .172 |
| CABG | 2.8 | 2.4 | .673 | 4.6 | .358 | .316 |
| Acute medical treatment | ||||||
| Aspirin | 96.0 | 86.5 | < .001 | 86.1 | < .001 | .919 |
| P2Y12inhibitors | 83.9 | 61.3 | < .001 | 85.1 | .580 | < .001 |
| Clopidogrel | 64.2 | 52.9 | 59.6 | |||
| Prasugrel | 25.3 | 9.7 | 19.8 | |||
| Ticagrelor | 36.4 | 20.1 | 35.1 | |||
| GPIIb/IIIa inhibitors | 24.3 | 11.3 | < .001 | 20.5 | .013 | < .001 |
| Bivalirudin | 1.7 | 1.7 | .842 | 1.3 | .657 | .632 |
| Beta-blockers | 61.9 | 61.2 | .934 | 54.2 | .026 | .038 |
| Nitrates | 51.5 | 48.4 | .014 | 54.1 | .367 | .066 |
| ACE inhibitor/ARB | 54.6 | 49.3 | < .001 | 55.7 | .692 | < .001 |
| Ca2+channel blockers | 8.7 | 12.4 | < .001 | 9.5 | .996 | .147 |
| Diuretics | 20.4 | 47.2 | < .001 | 37.8 | < .001 | .002 |
| Statins | 76.8 | 60.2 | < .001 | 67.8 | < .001 | .013 |
| Vasopressors | 7.4 | 11.3 | < .001 | 17.7 | < .001 | .002 |
| Discharge therapy | ||||||
| Aspirin | 96.9 | 81.1 | < .001 | 88.6 | < .001 | .004 |
| P2Y12inhibitors | 86.5 | 62.2 | < .001 | 77.3 | < .001 | < .001 |
| Clopidogrel | 63.8 | 54.3 | < .001 | 66.4 | .365 | < .001 |
| Prasugrel | 28.6 | 9.2 | < .001 | 8.4 | < .001 | .741 |
| Ticagrelor | 31.6 | 12.5 | < .001 | 12.7 | < .001 | .961 |
| OAC | 6.2 | 43.4 | < .001 | 43.5 | < .001 | .934 |
| Triple therapy | 3.6 | 18.7 | < .001 | 27.9 | < .001 | < .001 |
| OAC + any P2Y12 | 4.0 | 21.4 | < .001 | 32.3 | < .001 | < .001 |
| Dual antiplatelet | 84.8 | 56.6 | < .001 | 71.4 | < .001 | < .001 |
| Beta-blockers | 79.1 | 78.6 | .099 | 74.4 | .012 | .121 |
| Nitrates | 0.8 | 13.9 | < .001 | 10.8 | < .001 | .161 |
| ACE inhibitor/ARB | 76.4 | 76.4 | .110 | 77.6 | .588 | .415 |
| Ca2+channel blockers | 10.0 | 14.6 | < .001 | 14.4 | .017 | .928 |
| Diuretics | 24.8 | 56.3 | < .001 | 53.5 | < .001 | .393 |
| Statins | 90.6 | 76.3 | < .001 | 83.9 | < .001 | .005 |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CABG, coronary artery bypass graft; GPIIb/IIIa, glycoprotein IIb/IIIa; IABP, intra-aortic balloon pump; LVEF, left ventricular ejection fraction; OAC, oral anticoagulant; PCI, percutaneous coronary intervention.
Data are expressed as No. (%).
A, B: trends of referrals for CA and PCI according to time of onset of atrial fibrillation. C, D: trends in prescription of triple therapy (OAC + DAPT) or OAC + any antiplatelet (OAC + SAP, either aspirin or any P2Y12 inhibitor) at discharge according to the time of onset of AF. AF, atrial fibrillation; CA, coronary angiography; DAPT, dual antiplatelet therapy; PCI, percutaneous coronary intervention; pre-AF, pre-existing atrial fibrillation; new-AF, new-onset atrial fibrillation; OAC, oral anticoagulant; SAP, single antiplatelet.
In pre-AF patients, acute management with aspirin, P2Y12 inhibitors, glycoprotein IIb/IIIa inhibitors, angiotensin-converting enzyme inhibitors/receptor blockers and beta-blockers were less frequently adopted than in non-AF patients. In contrast, vasopressors and symptomatic treatments with Ca2+ channel blockers and diuretics were frequently prescribed.
No differences in terms of referral for invasive diagnostics and PCIs were observed between new-AF and non-AF patients. As for patients with pre-AF, a trend toward an increased referral to coronary angiography and PCIs was evident during the study period. Also, in this case, femoral access was chosen in most patients. Hemodynamic support with vasopressors or balloon counterpulsation was relatively common in new-AF patients, needed in almost 1 out of 5. Evidence of severe left ventricular dysfunction was more frequent in patients with pre-AF (11.2%) and new-AF (13.3%) than in those without the arrhythmia (5.9%, P < .001 for both AF groups vs non-AF).
Acute management with P2Y12 inhibitors was comparable among new-AF patients and those without AF.
Aspirin was prescribed to most patients, while a dual antiplatelet regimen was less frequently prescribed both in pre-AF (56.6%) and in new-AF patients (71.4%) compared with non-AF patients (P < .001 for both). Nonetheless, a progressive increase in triple therapy counterbalanced by a decline in the use of any single antiplatelet + oral anticoagulation was evident for pre-AF but not new-AF (P for trends .002 and .36 respectively; Figure 2C and D).
In both AF subgroups, an oral anticoagulant was prescribed in only about 40% of patients (and almost a quarter were discharged to secondary care facilities while on iv/subcutaneous heparin). Both triple therapy (ie, the association between aspirin + any P2Y12 inhibitor + oral anticoagulant) and a dual antiplatelet regimen were prescribed more often in patients with new-AF than in patients with known AF (27.9% vs 18.7%, P < .001 and 71.4% vs 56.6%, P < .001, respectively). Beta-blockers were less frequently used in patients with AF, while prescriptions of symptomatic medications such as nitrates and diuretics were significantly higher when compared with non-AF patients.
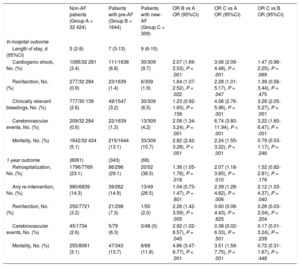
In-hospital OutcomeTable 3 reports details on in-hospital and 1-year outcome.
In-hospital and 1-year Outcome
| Non-AF patients (Group A = 32 424) | Patients with pre-AF (Group B = 1644) | Patients with new-AF (Group C = 309) | OR B vs A OR (95%CI) | OR C vs A OR (95%CI) | OR C vs B OR (95%CI) | |
|---|---|---|---|---|---|---|
| In-hospital outcome | ||||||
| Length of stay, d (95%CI) | 5 (2-8) | 7 (3-13) | 9 (6-15) | |||
| Cardiogenic shock, No. (%) | 1095/32 281 (3.4) | 111/1638 (6.8) | 30/309 (9.7) | 2.07 (1.69-2.53), P < .001 | 3.06 (2.09-4.48), P < .001 | 1.47 (0.96-2.25), P = .069 |
| Reinfarction, No. (%) | 277/32 284 (0.9) | 23/1639 (1.4) | 6/309 (1.9) | 1.64 (1.07-2.52), P = .022 | 2.28 (1.01-5.17), P = .047 | 1.39 (0.56-3.44), P = .475 |
| Clinically relevant bleedings, No. (%) | 777/30 139 (2.6) | 49/1547 (3.2) | 30/309 (6.5) | 1.23 (0.92-1.65), P = .156 | 4.06 (2.76-5.96), P < .001 | 3.28 (2.05-5.27), P < .001 |
| Cerebrovascular events, No. (%) | 209/32 284 (0.6) | 22/1639 (1.3) | 13/309 (4.2) | 2.08 (1.34-3.24), P = .001 | 6.74 (3.80-11.94), P < .001 | 3.22 (1.60-6.47), P = .001 |
| Mortality, No. (%) | 1642/32 424 (5.1) | 215/1644 (13.1) | 33/309 (10.7) | 2.82 (2.42-3.28), P < .001 | 2.24 (1.55-3.22), P < .001 | 0.79 (0.53-1.17), P = .246 |
| 1-year outcome | (8061) | (343) | (68) | |||
| Rehospitalization, No. (%) | 1796/7769 (23.1) | 86/296 (29.1) | 20/52 (38.5) | 1.36 (1.05-1.76), P = .018 | 2.07 (1.18-3.65), P = .010 | 1.52 (0.82-2.81), P = .176 |
| Any re-intervention, No. (%) | 980/6839 (14.3) | 39/262 (14.9) | 13/49 (26.5) | 1.04 (0.73-1.47), P = .801 | 2.39 (1.28-4.62), P = .006 | 2.12 (1.03-4.37), P = .040 |
| Reinfarction, No. (%) | 250/7721 (3.2) | 21/298 (7.3) | 1/50 (2.0) | 2.26 (1.42-3.59), P = .005 | 0.60 (0.08-4.43), P = .625 | 0.26 (0.03-2.04), P = .204 |
| Cerebrovascular events, No. (%) | 45/1734 (2.6) | 5/79 (6.3) | 0/48 (0) | 2.92 (1.02-6.57), P = .045 | 0.38 (0.02-6.33), P = .501 | 0.17 (0.01-3.24), P = .239 |
| Mortality, No. (%) | 255/8061 (3.1) | 47/343 (13.7) | 8/68 (11.8) | 4.86 (3.47-6.77), P < .001 | 3.51 (1.59-7.75), P = .001 | 0.72 (0.31-1.67), P = .448 |
95%CI, 95% confidence interval; AF, atrial fibrillation; new-AF, new-onset AF; OR, odds ratio; pre-AF, pre-existing atrial fibrillation.
Pre-AF patients had a longer hospital stay (7 interquartile range [3-13] vs 5 interquartile range [2-8] days; P < .001) and showed higher rates of in-hospital complications such as cardiogenic shock (OR, 2.07; 95%CI, 1.69-2.53; P < .001), reinfarction (OR, 1.64; 95%CI, 1.07-2.52; P = .022), and cerebrovascular events (OR, 2.08; 95%CI, 1.34-3.24; P = .001), while no differences in clinically relevant bleedings (OR, 1.23; 95%CI, 0.92-1.65; P = .156) were evident when compared with non-AF patients. Crude in-hospital mortality was also higher (non-AF 5.1% vs pre-AF 13.1%; OR, 2.82; 95%CI, 2.24-3.28; P < .001).
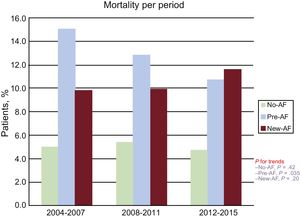
Between the first and last third of the study period analyzed (ie, 2004-2007 and 2015) in-hospital mortality declined from 15.0% to 10.7% in pre-AF patients (P for trend = .035).
New-AF patients showed the highest rate of in-hospital bleedings and cerebrovascular events among the 3 groups. In-hospital mortality among both AF groups was comparable (new-AF vs pre-AF [OR, 0.79; 95%CI, 0.57–1.17; P = .246]).
In-hospital mortality remained substantially stable in new-AF patients throughout the whole study period (P for trend = .42). Figure 3 describes trends of in-hospital mortality according to the presence and type of AF during the first, second and last third of the study period.
Outcome at Follow-upIn the subpopulation of patients scheduled for 1-year follow-up (n = 8959; 24.9% of the entire population), those with AF at admission had more rehospitalizations for any cardiovascular reason (OR, 1.36; 95%CI, 1.07-1.76; P = .01), reinfarction (OR, 2.26; 95%CI, 1.42-3.59; P = .001) and cerebrovascular events (OR, 2.92; 95%CI, 1.02-6.57; P = .045), while no significant differences were evident in terms of recurrent invasive procedures such as angiographies, PCI, bypass surgery, or pacemaker/implantable cardioverter-defibrillator implantation (OR, 1.04; 95%CI, 0.73-1.47; P = .801) compared with non-AF patients. One year mortality in pre-AF was 4 times higher than that of non-AF patients (13.7% vs 3.1%; OR, 4.86; 95%CI, 3.47-6.77; P < .001).
At 1-year follow-up, more than 1 out of 3 patients with episodes of AF during the index admission had a new hospitalization for any cardiovascular reason (OR, 2.07; 95%CI, 1.18-3.65; P = .01 vs non-AF patients) and referrals for invasive procedures (OR, 2.39; 95%CI, 1.28-4.62; P = .006) but rates of recurrent cardiac ischemic events (OR, 0.60; 95%CI, 0.08-4.43; P = .625) and cerebrovascular events (OR, 0.38; 95%CI, 0.02-6.33; P = .501) were similar to those observed in non-AF patients.
No strokes were evident in new-AF patients during follow-up. One year mortality was 11.8%, higher than that observed in non-AF patients (OR, 3.51; 95%CI, 1.59-7.75; P = .001), but comparable to that in pre-AF patients (OR, 0.72; 95%CI, 0.31-1.67; P = .625).
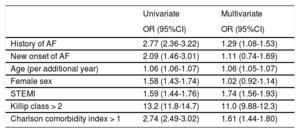
Outcome PredictorsTable 4 shows the results of the univariable and multivariable analyses.
Independent Predictors of In-hospital Mortality in Acute Coronary Syndrome Patients
| Univariate | Multivariate | |
|---|---|---|
| OR (95%CI) | OR (95%CI) | |
| History of AF | 2.77 (2.36-3.22) | 1.29 (1.08-1.53) |
| New onset of AF | 2.09 (1.46-3.01) | 1.11 (0.74-1.69) |
| Age (per additional year) | 1.06 (1.06-1.07) | 1.06 (1.05-1.07) |
| Female sex | 1.58 (1.43-1.74) | 1.02 (0.92-1.14) |
| STEMI | 1.59 (1.44-1.76) | 1.74 (1.56-1.93) |
| Killip class > 2 | 13.2 (11.8-14.7) | 11.0 (9.88-12.3) |
| Charlson comorbidity index > 1 | 2.74 (2.49-3.02) | 1.61 (1.44-1.80) |
95%CI, 95% confidence interval; AF, atrial fibrillation; OR, odds ratio; STEMI, ST-segment elevation myocardial infarction.
All the above variables were used in the adjusted model.
A multivariable analysis was performed that included history of AF, intercurrent episodes of AF, age, female sex, STEMI presentation, Killip class > 2 at admission and Charlson comorbidity index > 1. History of AF (OR, 1.29; 95%CI, 1.08-1.53; P = .005), age (OR per additional year, 1.06; 95%CI, 1.05-1.07; P < .001), STEMI presentation (OR, 1.74; 95%CI, 1.56-1.93; P < .001), Killip class > 2 (OR, 11.0; 95%CI, 9.88-12.3; P < .001) and Charlson Comorbidity index > 1 (OR, 1.61; 95%CI, 1.45-1.80; P < .001) were independent predictors of in-hospital mortality, while new-onset AF during hospitalization was not independently associated with in-hospital mortality (OR, 1.02; 95%CI, 0.74-1.69; P = .614).
DISCUSSIONTo the best of our knowledge, this is the largest European contemporary registry evaluating the relative impact on morbidity and mortality of pre-existing vs new-onset AF in the setting of an ACS.
The following key messages can be drawn from our data:
- •
Despite different baseline clinical profiles, patients with pre-existing AF and those with new-onset AF both experienced high and comparable in-hospital and 1-year mortality.
- •
Pre-existing AF but not new-onset AF was an independent predictor of in-hospital mortality.
- •
New-onset AF, being frequently associated with STEMI presentation, out of hospital cardiac arrest and risk of hemodynamic deterioration, was a marker of worse hemodynamic impact of ACS.
- •
During the 10 years evaluated, a progressive increase in referrals to PCI with a concomitant reduction of mortality was observed in patients with pre-existing AF. In contrast, mortality remained substantially stable and high in patients with new-onset AF.
Previous works have analyzed the clinical characteristics and prognostic impact of known and de novo AF associated with an ACS. Data derived from the GRACE/CANRACE and Medicare registries showed that the incidence of known AF ranged from 7.6% to 22.1% in ACS patients. AF patients were older, with an increased burden of cardiovascular risk factors as well as known coronary disease, heart failure, and renal impairment, with lesser access to evidence-based therapies and a noteworthy in-hospital mortality.2,5,10 Moreover, the paradigm that pre-existing compared with newly discovered AF had a different mode of onset, pathophysiology, and in-hospital and long-term outcomes was emphasized and a prognostic role for newly developed AF was also hypothesized.5
Our data propose a comprehensive interpretation for both types of AF. Despite different clinical characteristics and pathophysiological mechanisms, the comparable and high in-hospital and 1-year mortality observed in both AF groups unifies those 2 entities from a prognostic perspective. Independently from different modes and timing of onset, both AF groups showed a high and comparable in-hospital mortality, this representing a marker of clinical interest.
There is conflicting evidence on the relative role of the 2 different AF forms. While some authors do not recognize a clear role as a predictor of in-hospital mortality for pre-existing AF2,5,16,17 the worse in-hospital outcome not being attributed to a direct role of AF but rather to the associated prognosticators such as age or poorer overall clinical status at presentation, others postulate a causative effect.8,9
Our analysis shows that pre-existing AF, but not new-onset AF, was an independent predictor of in-hospital mortality. This finding highlights the role of the arrhythmia as a prognosticator in ACS, to be potentially considered in association with currently recommended–or in the development of future–risk estimation scores.18,19 On the other hand, the occurrence of new episodes of AF was frequently associated with STEMI presentation, out of hospital cardiac arrest, hemodynamic deterioration requiring mechanical or pharmacological support, underscoring the role of the hemodynamic impact of the ACS as a trigger for AF.20 Those associations were considered as the factors leading to the lack of significance for new-AF as a predictor of in-hospital mortality in our population. Nonetheless, regardless of statistical nonsignificance, the clinical importance of the new episodes of AF in the setting of AF remains. In fact, despite a younger age and a lower burden of associated comorbidities observed in patients with new onset of the arrhythmia, the hemodynamic impact of ACS overrides their better baseline overall status driving morbidity/mortality.
Trend analysis clearly underlined a progressive decline of in-hospital mortality for pre-existing AF patients. This confirms a tendency evident in the literature reporting in-hospital and 1-year deaths exceeding 20% and 40% in the mid 1990s,5 15% and 30% in early 2000,9 and 10% in more contemporary series.7,10 While this trend was clear in patients with pre-existing AF, mortality remained stable and high in patients with new-onset AF, strengthening once more the clinical importance of this entity.
Furthermore, while no causative relation can be drawn from our data, between the first and last year analyzed (ie, 2004 and 2014) the decline of in-hospital mortality observed in patients with pre-existing AF was mirrored by an increased referral for PCI. This represents an interesting hypothesis-generating finding, potentially related to the recent improvements in interventional tools and techniques (such as the adoption of radial access or third generation stents allowing shortened antiplatelet therapies) that might further enhance the benefits of percutaneous revascularization even in frail patients.
Beside prognostic implications, physicians are called to face difficult therapeutic decisions when managing AF during the acute phase of a coronary ischemic event, and have to balance the embolic and hemorrhagic risks; these decisions are often based on expert consensus, due to the lack of evidence-based confirmations.1,21,22
Our data clearly show that multiple therapeutic regimens are adopted, potentially leading to misleading impacts on morbidity and mortality. Both underuse of anticoagulant/antiplatelet therapies and revascularizations in patients with known AF may explain the observed increased incidence of reinfarction and cerebrovascular events at 1-year.In addition, in the setting of new-AF, increased rates of in-hospital bleedings and cerebrovascular events were observed, probably due to an overestimation of their embolic risk.
The adoption of proven measures known to be effective in reducing hemorrhagic complications and mortality such as the radial should be further encouraged.23
Strengths and LimitationsSeveral strengths and limitations of our work should be mentioned.
This analysis, evaluating the relationships between AF and ACS, derived from the data of the Swiss national registry, provides a western updated overview allowing us to depict a national real-life scenario and to provide clues for optimization of current standards of care. Nonetheless, as an observational nonrandomized investigation, our data are subject to certain limitations such as missing data and patient selection or other unforeseen confounders. First, the large number of our population with AF both and admission as well as those with intercurrent episodes is a limitation to the external validity of our observations. While vital status at discharge was available for the whole set of enrolled patients, follow-up was available for a large portion but not for the whole population, thus allowing us to report only an estimate of postdischarge morbidity/mortality. Moreover, the progresses of medical/interventional treatments are mirrored by the transformations of the registry. Even in this relatively short observation period, some major innovations have been introduced such as standardized definitions for in-hospital complications or data regarding treatments that are available only in a proportion of our patients. Finally, the registry design did not allow us to classify patients according to the type of AF (whether paroxysmal, persistent or chronic), or to exclude the possibility that a certain percentage of patients classified as new-AF might have had an arrhythmia onset close to the hospital admission, thus being potentially ACS-related. Equally, we did not provide any data about the outcome of rhythm disturbance at follow-up.
CONCLUSIONSOur data confirm that AF in the setting of ACS is a major clinical problem. Both patients with history of AF and those with new-onset AF showed in-hospital complications and significantly increased and comparable in-hospital and 1-year mortality. While a history of AF is independently associated with in-hospital mortality, new-onset AF episodes may mirror a worse hemodynamic impact of the ACS, this ultimately driving the prognosis. Despite different clinical characteristic, burden of associated comorbidities and pathophysiological mechanism and in-hospital morbidity, pre-existing and new-onset AF share high and comparable acute and long-term mortality.
FUNDINGThe AMIS Plus registry is funded by unrestricted grants from the Swiss Heart Foundation and from Abbot AG, Amgen AG, AstraZeneca AG, Bayer (Schweiz) AG, Biotronik AG, Boston Scientific AG, B. Braun Medical AG, Daiichi-Sankyo /Lilly AG, GE Healthcare AG, Johnson & Johnson AG – Cordis Division, Medtronic AG, A. Menarini AG, Merck Sharp & Dohme AG, Mepha Pharma AG, Novartis Pharma Schweiz AG, Sanofi-Aventis (Schweiz) AG, Servier (Suisse) AG, St. Jude Medical (Schweiz) AG, Vascular Medical GmbH; all in Switzerland. The sponsors did not play any role in the design, data collection, analysis, or interpretation of the registry.
CONFLICTS OF INTEREStNone declared.
- -
In the setting of ACS, patients with AF are known to have a worse prognosis and lesser access to invasive treatments. Nonetheless, it is still a matter of debate whether AF adversely affects prognosis in ACS per se or is a marker of comorbidities, which effectively drive the outcome. Moreover, the relative impact of pre-existing compared with new-onset AF is debated.
- -
Despite being intrinsically different from a pathophysiological perspective, pre-existing and new-onset AF showed a high and comparable in-hospital and 1-year mortality. While pre-existing AF was an independent predictor of in-hospital mortality, new-onset AF, being frequently associated with STEMI presentation, out of hospital cardiac arrest and risk of hemodynamic deterioration, was interpreted as a marker of worse hemodynamic impact of the ACS.
.