Coronary heart disease is the leading cause of heart failure (HF). The aim of this study was to assess the risk of readmission for HF in patients with acute coronary syndrome without previous HF or left ventricular dysfunction.
MethodsProspective study of consecutive patients admitted for acute coronary syndrome in 2 institutions. Risk factors for HF were analyzed by competing risk regression, taking all-cause mortality as a competing event.
ResultsWe included 5962 patients and 567 (9.5%) experienced at least 1 hospital readmission for acute HF. Median follow-up was 63 months and median time to HF readmission was 27.1 months. The cumulative incidence of HF was higher than mortality in the first 7 years after hospital discharge. A higher risk of HF readmission was associated with age, diabetes, previous coronary heart disease, GRACE score> 140, peripheral arterial disease, renal dysfunction, hypertension and atrial fibrillation; a lower risk was associated with optimal medical treatment. The incidence of HF in the first year of follow-up was 2.73% and no protective variables were found. A simple HF risk score predicted HF readmissions risk.
ConclusionsOne out of 10 patients discharged after an acute coronary syndrome without previous HF or left ventricular dysfunction had new-onset HF and the risk was higher than the risk of mortality. A simple clinical score can estimate individual risk of HF readmission even in patients without previous HF or left ventricular dysfunction.
Keywords
The incidence of chronic heart failure (HF) has increased exponentially in the last few decades1,2 and therefore the detection of risk factors related to its incidence should be a primary target for research.3 Coronary heart disease is the leading cause of HF.1,4 Acute coronary syndrome (ACS) is the most common presentation of coronary heart disease and produces the greatest myocardial damage. The presence of myocardial infarction, either as an ACS or as a silent event, increases the long-term incidence of HF4,5 up to 30%.
In-hospital HF is one of the strongest predictors of the incidence of postdischarge HF, regardless of left ventricular ejection fraction (LVEF)6; nonetheless, most ACS patients do not have left ventricular dysfunction or HF6–9 and there is scarce evidence of risk factors related to the incidence of long-term HF or the most protective treatments,5,7 possibly because the incidence is expected to be low. A risk score that identifies patients at higher risk of HF after an ACS has recently been proposed but it includes previous episodes of HF and LVEF <0.50.10
The aim of the current study was to describe the incidence and risk factors for the short- and long-term incidence of HF in large cohort of ACS patients without previous HF or left ventricular dysfunction.
METHODSStudy designWe performed a prospective study of all consecutive patients admitted for ACS to 2 different centers between 2006 and 2016. A total of 8771 patients were admitted for ACS between November 2003 and December 2016. We excluded patients who died within the hospitalization (n=450), those who developed HF during hospitalization (n=1641), patients diagnosed with HF before the ACS hospitalization (n=349), and those with LVEF <0.50 measured in the index ACS. Finally, 5237 patients were included in the current study (figure 1 of the supplementary data). ACS was defined as the presence of typical clinical symptoms of chest pain and electrocardiographic changes indicative of myocardial ischemia/lesion and/or elevation of serum markers of myocardial damage.11
The primary endpoint was the incidence of a first HF hospitalization and the secondary endpoint was the incidence of HF in the first year after the ACS. Diagnosis of HF was codified according to medical reports, signed by the medical staff of each institution, and was mainly based on the diagnostic criteria in clinical guidelines.12 ACS was classified as ST-segment elevation myocardial infarction and non–ST-segment elevation ACS according to the electrocardiographic findings. Mortality risk was assessed by the GRACE score11 and patients were categorized, according to current recommendations, into low (< 108), intermediate (109-139), or high risk (> 140). We assessed the risk of HF readmission according to the CardioCHUS-SanJuan HF score10; patients were divided into the 3 risk categories proposed in the original report (< 9: low risk; 9-15: intermediate risk;> 15 high risk of HF readmission). The completeness of revascularization was prospectively determined after the revascularization procedure, using the criterion of intended “equivalent anatomic” revascularization prior to the procedure based on segment numbering of vessels with a diameter> 1.5mm.10
Risk factors, clinical antecedents, treatments, complementary tests and main diagnosis at discharge were collected from all patients by trained medical staff. The diagnostic and therapeutic ACS protocols in both centers include blood sample determinations in the emergency department and the first fasting state after hospital admission. Glomerular filtration rate was estimated from serum creatinine values with the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation. For the antecedent of previous coronary heart disease, patients needed to have a clinical diagnosis of myocardial infarction, stable or unstable angina, or angina-driven coronary revascularization. Previous HF was codified if patients had at least 1 hospitalization with this main diagnosis in the discharge medical report as well as those with typical signs and symptoms of HF who had a compatible imaging diagnosis (x-ray or echocardiogram). Comorbidities were assessed by the Charlson index, adapted for patients with cardiovascular disease,13 and patients with a Charlson score> 4 qualified for high-comorbidity burden. According to current recommendations, optimal medical treatment was codified when patients received these 4 treatments jointly: antiplatelets, statins, beta-blockers, and an angiotensin-converter enzyme inhibitor or angiotensin-receptor blocker.14,15
The postdischarge follow-up of patients is specified in a well-established protocol in each center and is performed by telephone calls, and review of electronic medical reports and institutional databases. Vital status was verified by telephone calls in the absence of medical reports. The study protocol was approved by the ethics committee of the coordinating hospital.
Statistical analysisQuantitative variables are presented as mean±SD and differences were assessed by the Student t-test and chi-square test. Qualitative variables are presented as percentages and differences were analyzed by the ANOVA test. Survival analyses were performed after verification of the proportional risk assumption by the Schoenfied residuals test. The incidence of postdischarge HF could be affected by patients’ death and, therefore, the usual techniques for time-to-event analysis would provide biased or uninterpretable results due to the presence of competing risks and the Kaplan-Myer estimation could overestimate the real HF incidence.10,16 To avoid such effects, we applied the model introduced by Fine and Gray17 to test for competing events. The incidence of HF is presented in cumulative incidence function graphs and the results of multivariate analysis as subhazard ratio (sHR) and corresponding 95%CI. Harrelĺs c-statistic test was used to assess the discrimination of the model meanwhile calibration was tested by the Gronnesby and Borgan test. Patients lost to follow-up were categorized as missing, as well as those who lacked any of the main variables for the analyses, although these were very few. The predictive capacity of the CardioCHUS-SanJuan HF risk score10 was tested by the area under the curve of the receiver operator curve of the score.
Statistical difference was accepted at P <.05. All analyses were performed using STATA 14.3 (StataCorp. 2009. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
RESULTSWe included 5962 patients discharged from hospital after an ACS and 567 (9.5%; 95%CI, 8.3-9.8) experienced at least 1 hospital readmission for acute HF during follow-up. The clinical features of the cohort are presented in table 1. Patients who developed HF had higher mean age, were more frequently female, and had a higher prevalence of cardiovascular risk factors and comorbidities. With regard to the index ACS, patients who developed postdischarge HF less frequently had ST-segment elevation myocardial infarction but had higher GRACE scores. The mean CardioCHUS-SanJuan HF risk score was statistically significantly higher in patients who developed HF. Small but significant differences were also observed in medical treatments at discharge.
Clinical features of the cohort according the whether patients developed new-onset heart failure (HF) or not
| Total | No HF | HF | P | |
|---|---|---|---|---|
| No. | 5962 | 5395 (90.5) | 567 (9.5) | |
| Age | 65.5±12.6 | 64.5±12.6 | 74.8±8.9 | <.001 |
| Female sex, % | 25.9 | 23.7 | 46.6 | <.001 |
| Diabetes, % | 26.1 | 23.7 | 45.9 | <.001 |
| Hypertension, % | 57.9 | 55.7 | 79.2 | <.001 |
| Current smokers, % | 28.4 | 29.8 | 15.0 | <.001 |
| Dyslipidemia, % | 49.4 | 48.8 | 55.2 | .004 |
| Previous CHD, % | 19.5 | 18.2 | 31.6 | <.001 |
| Peripheral arterial disease, % | 6.5 | 5.6 | 15.3 | <.001 |
| Previous stroke, % | 5.0 | 4.6 | 8.5 | <.001 |
| Atrial fibrillation, % | 7.3 | 6.1 | 18.1 | <.001 |
| COPD, % | 8.0 | 7.0 | 17.6 | <.001 |
| STEMI, % | 28.8 | 29.6 | 21.5 | <.001 |
| GRACE score | 135.9±34.8 | 133.5±33.9 | 156.0±35.2 | <.001 |
| GRACE score> 140, % | 38.7 | 36.1 | 63.0 | <.001 |
| CRUSADE score | 19.0±15.5 | 17.5±14.4 | 30.7±19.1 | <.001 |
| Charlson score | 2.1±2.1 | 2.0±2.0 | 3.2±2.5 | <.001 |
| Charlson score> 4, % | 17.2 | 15.4 | 32.0 | <.001 |
| LVEF, % | 61.2±4.7 | 61.2±4.6 | 61.2±3.0 | .931 |
| Angiography, % | 92.8 | 93.4 | 87.5 | <.001 |
| Revascularization, % | 74.9 | 76.0 | 64.4 | <.001 |
| Complete revascularization, % | 50.1 | 51.7 | 34.9 | <.001 |
| Hemoglobin, g/dL | 14.0±3.1 | 14.1±2.4 | 13.3±6.4 | <.001 |
| Total cholesterol, mg/dL | 177.6±46.0 | 178.4±45.9 | 169.4±46.2 | <.001 |
| LDL-C cholesterol, mg/dL | 108.2±39.0 | 108.9±39.0 | 101.5±37.6 | <.001 |
| Fasting glucose, mg/dL | 137.5±125.4 | 134.2±127.8 | 165.3±94.4 | <.001 |
| Creatinine, mg/dL | 1.0±1.1 | 1.0±1.2 | 1.2±0.7 | <.001 |
| GFR mL/min/1.72 m2 | 77.7±22.) | 79.3±21.8 | 62.1±22.4 | <.001 |
| GFR <60 mL/min/1.72 m2, % | 21.0 | 18.4 | 46.9 | <.001 |
| CardioCHUS-SanJuan HF score | 3.6±7.7 | 2.8±7.2 | 11.0±7.5 | <.001 |
| Medical treatments at discharge | ||||
| Aspirin, % | 91.1 | 91.6 | 86.2 | <.01 |
| Clopidogrel, % | 63.0 | 62.7 | 65.9 | .134 |
| Ticagrelor, % | 5.8 | 6.3 | 1.2 | <.01 |
| Prasugrel, % | 3.4 | 3.7 | 0.9 | <.01 |
| DAPT, % | 69.9 | 70.3 | 65.9 | .030 |
| Oral anticoagulation, % | 5.9 | 5.0 | 15.0 | <.01 |
| ACEI/ARB, % | 63.3 | 63.1 | 65.7 | .06 |
| Beta-blockers, % | 72.9 | 74.1 | 59.7 | <.01 |
| Diuretics, % | 12.5 | 10.3 | 34.1 | <.01 |
| Statins, % | 86.5 | 86.9 | 82.5 | <.01 |
| Mineralcorticoid antagonists, % | 1.5 | 1.3 | 3.4 | <.01 |
| Insulin/oral antidiabetics, % | 17.4 | 16.0 | 30.2 | <.01 |
ACEI, angiotensin-converter enzyme inhibitors; ARB, angiotensin-receptor blocker; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; DAPT, dual antiplatelet treatment; GFR, glomerular filtration rate; HF, heart failure; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricle ejection fraction; STEMI, ST-segment elevation myocardial infarction.
Unless otherwise indicated, the data are expressed as No. (%) or mean±standard deviation.
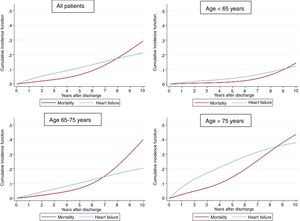
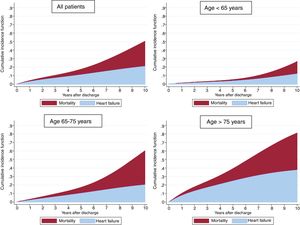
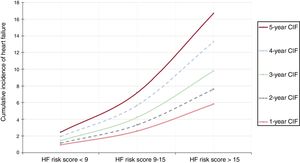
Follow-up was available in 98.4% of the cohort with a median follow-up of 63.0 [31-82] months. A total of 998 (16.7%) patients died, with 625 (10.5%) of cases being attributable to cardiovascular causes. Median time to HF readmission was 31 [8-53] months. As shown in figure 1, the cumulative incidence of HF showed a linear increase and was higher than mortality in the first 7 years after the index ACS; the cumulative incidence of mortality was fairly low in the first years after hospital discharge.
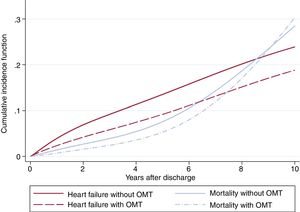
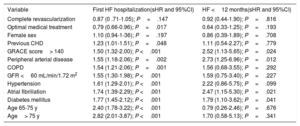
Table 2 shows the results of the competing risk regression, adjusted by age, sex, risk factors, previous cardiovascular disease, medical treatments, LVEF, and revascularization. Many risk factors were associated with a higher risk of HF; age was the leading risk factor and the effect intensified as age increased (figure 2); the only variable associated with a lower risk of HF was optimal medical treatment (figure 3). The model had a good discrimination capacity (Harell's c-statistic 0.81; 95%CI, 0.79-0.82; P <.001) and was accurately calibrated (P=.71). figure 4
Results of the competing risk regression analysis for heart failure (HF) incidence
| Variable | First HF hospitalization(sHR and 95%CI) | HF <12 months(sHR and 95%CI) |
|---|---|---|
| Complete revascularization | 0.87 (0 .71-1.05); P=.147 | 0.92 (0.44-1.90); P=.816 |
| Optimal medical treatment | 0.79 (0.66-0.96); P=.017 | 0.64 (0.33-1.25); P=.193 |
| Female sex | 1.10 (0.94-1-36); P=.197 | 0.86 (0.39-1.89); P=.708 |
| Previous CHD | 1.23 (1.01-1.51); P=.048 | 1.11 (0.54-2.27); P=.779 |
| GRACE score> 140 | 1.50 (1.32-2.00); P <.001 | 2.52 (1.13-5.65); P=.024 |
| Peripheral arterial disease | 1.55 (1.18-2.06); P=.002 | 2.73 (1.25-6.96); P=.012 |
| COPD | 1.54 (1.21-2.06); P=.001 | 1.56 (0.68-3.55); P=.292 |
| GFR <60 mL/min/1.72 m2 | 1.55 (1.30-1.98); P <.001 | 1.59 (0.75-3.40); P=.227 |
| Hypertension | 1.61 (1.29-2.01); P <.001 | 2.22 (0.86-5.75); P=.099 |
| Atrial fibrillation | 1.74 (1.39-2.29); P <.001 | 2.47 (1.15-5.30); P=.021 |
| Diabetes mellitus | 1.77 (1.45-2.12); P <.001 | 1.79 (1.10-3.62); P=.041 |
| Age 65-75 y | 2.40 (1.78-3.22); P <.001 | 0.79 (0.26-2.46); P=.676 |
| Age> 75 y | 2.82 (2.01-3.87); P <.001 | 1.70 (0.58-5.13); P=.341 |
95%CI, 95% confidence interval; CHD, coronary heart disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HF: heart failure; sHR, sub-hazard ratio.
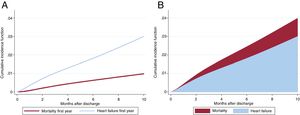
We assessed the incidence of HF in the first year of follow-up. Within this period, 163 patients (2.73%; 95%CI, 2.31-3.14) were admitted for acute HF while mortality was <1%. Median time to readmission was 111 [37-218] days. As shown in table 2, diabetes, atrial fibrillation, GRACE score> 140 and peripheral disease were the variables with higher risk of HF in the first year after the ACS and no protective variables were identified. The model had even better discrimination capacity (Harells’ c-statistic 0.84; 95%CI, 0.77-0.90; P <.001) and was accurately calibrated (P=.69). The area under the curve for the CardioCHUS-SanJuan HF risk score (figure 2 of the supplementary data) for the prediction of first HF readmission was 0.78 (95%CI, 0.76-0.79; P <.01) and that for readmission within the first year was 0.79 (95%CI, 0.77-0.82).
Since most clinical variables associated with HF readmission were included in the CardioCHUS-SanJuan HF risk score, we assessed the incidence of HF risk categories. As shown in figure 5, there was a gradual increase in the incidence of the yearly incidence of HF readmission in each risk category. Multivariate analysis revealed that patients with a CardioCHUS-SanJuan HF risk score 9-15 (sHR, 1.51; 95%CI, 1.20-1.90; P <.001) and, especially those with a CardioCHUS-SanJuan HF risk score> 15 (sHR, 2.38; 95%CI, 1.83-3.09), had an increased risk of HF readmission.
DISCUSSIONThe objective of our study was to describe new-onset HF in patients discharged after an ACS, who are usually considered at low risk of HF as they do not have left ventricular dysfunction or previous HF. Nonetheless, the long-term follow-up in our study highlights that 10% of these patients developed new-onset HF and that many variables were positively associated with this highly important complication. HF within the first year after discharge was less common and was linked to just a few risk factors. Since clinical features and mortality rates were similar to those in previous reports,4,8,9,18–23 we believe that our results might be representative of daily clinical practice. We also believe that our results have important implications both in the acute and in the long-term management of patients admitted for ACS.
The incidence and prevalence of HF has increased in the last decades due to multiple variables, such as longer life expectancy, the increase in risk factors,12,24,25 and reductions in in-hospital mortality among patients admitted for ACS; all these issues generate a growing population at high risk of recurrent events,4,7,10,26,27 recently named chronic coronary syndrome by the 2019 European Society of Cardiology guidelines.28 Therefore, the in-hospital and long-term management of ACS deserves maximum consideration to prevent subsequent complications and improve outcomes. Reductions in in-hospital mortality as well as revascularization or reperfusion have been clearly established as quality markers of ACS treatment29 but recurrent events have a strong impact on prognosis and should also be taken into account. Recurrent nonfatal events strongly affect patients’ quality of life and, more interestingly, they increase mortality 2-fold in the following 2 years.30 This effect is much more relevant when the recurrent event is HF hospitalization because it is associated with a more than 4-fold higher risk of death.19,23
Our study shows that 1 out of 10 patients discharged with a diagnosis of an ACS will have at least 1 hospital readmission for HF in the first 5 years after the index ACS, which is much higher than the incidence of other complications, such as major bleeding8,31 or stroke.26 HF hospitalizations have a strong impact on patients’ family and resources12 and, therefore, deserve maximum consideration. Postdischarge specific follow-up protocols, such as Cardiac Rehabilitation programs32 or specialized units,27 have demonstrated benefits in risk factor control as well as in reducing mortality and readmission; we believe that our results might help to identify patients at higher risk of new-onset HF. Moreover, the CardioCHUS-SanJuan score has a reliable value for detecting patients at high risk of HF readmission.
We tested the diagnostic accuracy of a risk score specifically designed to predict HF readmission after an ACS.10 The area under the curve of the model in the original cohort, including patients with previous HF or left ventricular dysfunction, was 0.77 (95%CI, 0.76-0.78) and the results of our study were fairly similar, consistent with the original publication. A similar registry of post-ACS, also from Spain, proposed a risk based on 4 variables and had a similar preventive value (c-statistic 0.74).23 Both risk scores share variables, such as age, hypertension, and glomerular filtration rate <60mL/min/1.72 m2, although the CardioCHUS-SanJuan HF risk score also includes complete revascularization, diabetes, and optimal medical treatment. Nonetheless, the variables included in the other registry were those that obtained statistically significant results in the current study, which reinforces both scores and both reports. Moreover, we believe that this reflects a growing concern related to the incidence of post-ACS HF and the lack of reliable and contemporary evidence in this field.
Revascularization is the cornerstone of ACS treatment. Complete revascularization was included in the CardioCHUS-SanJuan HF risk score because it was independently associated with lower HF readmission but it was reproduced in the current analysis. ACS is the time of maximal myocardial damage and revascularization,11 beta-blockers,33 and angiotensin-receptor blockers34 have been demonstrated to reduce myocardial damage and left ventricular remodelling as well as to confer a survival benefit. Complete revascularization was not associated with lower HF readmission in this cohort of patients with LVEF> 0.50, in contrast with the original publication, which might reflect the less determinant effect in patients with less myocardial damage, at least in terms of subsequent HF.
In contrast, the effect of optimal medical treatment, understood as the combination of antiplatelets, statins, beta-blockers and angiotensin-receptor blocker or angiotensin-receptor blockers, was positively associated with a lower risk of HF readmission. Optimal medical treatment has been demonstrated to be efficient in major cardiovascular event reduction in patients with stable angina or chronic coronary heart disease14,15 and our results add the beneficial effect on HF readmission prevention.
Prevention of early HF onset after an ACS is a clinical challenge.12,23 Only 3.8% of our study population had a hospital readmission for HF in the first 12 months after ACS discharge, which reflects that the burden of HF admission should be assessed in the long-term; nonetheless, diabetes and peripheral arterial disease were identified as leading risk factors for premature HF readmission within the first year, which might reflect the presence of underlying cardiac damage before the ACS or a larger extent of damage during the ACS. A brand new family of antidiabetic drugs, SGLT-2 inhibitors, have provided robust evidence on HF readmission in high-risk patients with diabetes35 or even with HF and left ventricular dysfunction, regardless of diabetes mellitus36; our results might define a specific population who derive special benefit from these drugs. Patients with diabetes and HF have a very poor prognosis since the 5-year mortality rate is> 55%,37 reflecting an unmet need for effective therapies in these very-high risk patients. Very premature use of beta-blockers in ACS might increase the risk of cardiogenic shock or HF,33 which could explain the lack of benefit of beta-blockers on HF readmission in the first year.
Our study has some limitations. First, like all observational prospective studies, it has some inherent limitations such as the lack of randomization, long-term variations in medical treatments or uncontrolled variables, especially frailty, which has very important implications in HF or ACS patients.38 Onset of HF could also be underestimated, but since we analyzed only hospital readmissions we believe that we included real HF cases and did not take into account other cases of dyspnea or breathing disorders. Since the inclusion period was reasonably long, the use of ticagrelor and prasugrel was fairly low, which might now fairly represent current management of ACS, although there is no evidence on the role of antiplatelet treatment on the incidence of HF.39 Last, we assessed only the time to the first hospital readmission and did not analyze recurrent hospitalization for HF, which could have provided a more realistic view of the true prognosis.40 Since the clinical features and incidence of long-term events are similar to those of previous reports,4,8,9,18–23,41 we believe that the above-mentioned limitations might not have had an important impact on our results.
In conclusion, 1 out of 10 patients discharged after an ACS without previous HF or left ventricular dysfunction had at least 1 HF readmission. Complete revascularization and beta-blockers had a protective effect. A clinical score has a fair predictive capacity for the assessment of patients at higher risk of HF readmission after an ACS and could help the follow-up management of these patients.
CONFLICTS OF INTERESTThe authors declare that there are no conflicts of interest related to the results of this study.
- -
Coronary heart disease is the leading cause of HF.
- -
HF during hospitalization for ACS is the main determinant of subsequent HF.
- -
Onset of HF impairs prognosis and quality of life.
- -
One out of 10 patients will develop HF after ACS, despite not having left ventricular dysfunction or previous HF.
- -
The risk of HF is higher than the risk of death in these patients.
- -
Optimal medical treatment reduces the risk of HF in these patients.
The authors of this study received support from the Centro de Investigación Biomédica en Red de Enfermedades Cardiovasculares (CIBERCV CB16/11/00226 - CB16/11/00420), the national Spanish National Network for Biomedical Investigation on Cardiovascular Disease.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.03.011