Release kinetics of high-sensitivity cardiac troponin (hs-cTn) T and I in patients with acute myocardial infarction (AMI) are incompletely understood. We aimed to assess whether hs-cTnT/I release in early AMI is near linear.
MethodsIn a prospective diagnostic multicenter study the acute release of hs-cTnT and hs-cTnI within 1 and 2hours from presentation to the emergency department was quantified using 3 hs-cTnT/I assays in patients with suspected AMI. The primary endpoint was correlation between hs-cTn changes from presentation to 1 hour vs changes from presentation to 2hours, among all AMI patients and different prespecified subgroups. The final diagnosis was adjudicated by 2 independent cardiologists, based on serial hs-cTnT from the serial study blood samples and additional locally measured hs-cTn values.
ResultsAmong 2437 patients with complete hs-cTnT data, AMI was the adjudicated diagnosis in 376 patients (15%). For hs-cTnT, the correlation coefficient between 0- to 1-hour change and 0- to 2 hour change was 0.931 (95%CI, 0.916-0.944), P <.001. Similar findings were obtained with hs-cTnI (Architect) with correlation coefficients between 0- to 1-hour change and 0- to 2 hour change of 0.969 and hs-cTnI (Centaur) of 0.934 (P <.001 for both). Findings were consistent among type 1 and type 2 AMI and in the subgroup of patients presenting very early after chest pain onset.
ConclusionsPatients presenting with early AMI showed a near linear release of hs-cTnT and hs-cTnI. This near linearity provides the pathophysiological basis for rapid diagnostic algorithms using 0- to 1-hour changes as surrogates for 0- to 2 hour or 0- to 3 hour changes.
Registered at ClinicalTrials.gov (Identifier: NCT00470587).
Keywords
Patients with symptoms suggestive of acute myocardial infarction (AMI) account for about 10% of all emergency department (ED) consultations.1 Electrocardiogram (ECG) and cardiac troponin (cTn) T and I form the diagnostic cornerstones and complement clinical assessment.2,3
Recently developed high-sensitivity (hs) cTn assays provide a new noninvasive window to the heart and enable precise measurements of cTnT and cTnI blood concentrations around the 99th percentile of healthy individuals and even within the normal range, which was not possible with prior generations of tests.4–8 Their analytical superiority translated into clinical superiority in the early diagnosis of AMI.4–10 However, their analytical kinetics are yet not completely understood.
Pilot data generated in an experimental AMI model (alcohol septal ablation) and a registry provided the first hints that cTnT may be released in a near linear fashion in early AMI.11 Obtaining insights into the release kinetics of cTnT and cTnI from cardiomyocytes into the circulation within the first few hours of AMI would help to advance our understanding of AMI pathophysiology, including the temporal relationship between symptom onset and the development of cardiomyocyte necrosis and could possibly also help in the clinical implementation and adoption of recently developed rapid hs-cTn-algorithms.12–20 We therefore aimed to test the hypothesis that cTn release in early AMI is near linear in a large multicenter study.
METHODSStudy design and patient populationAdvantageous predictors of acute coronary syndrome evaluation (APACE) is an ongoing prospective international diagnostic multicenter study enrolling patients in 12 centers in 5 European countries (Switzerland, Spain, Italy, Poland, Czech Republic).5,21,22 The aim of APACE is to help to advance the early diagnosis of AMI. Adult patients presenting to the ED with symptoms suggestive of AMI with an onset or peak of symptoms within the last 12hours were enrolled after written informed consent was obtained.
Enrollment was independent from renal function, while patients with end-stage kidney failure on chronic dialysis were excluded. For this analysis, patients were also excluded if: a) measurements at presentation, after 1 hour, or after 2hours were not available for the respective hs-cTn assay investigated; b) the final diagnosis remained unclear even after adjudication and at least 1 hs-cTnT concentration was elevated (possibly indicating the presence of AMI); and c) the final diagnosis was other than AMI. Because some patients had missing data for some of the 3 investigational hs-cTn assays, 3 assay-specific subcohorts with a large overlap but numerically not identical sizes were derived from the main cohort.
The study was carried out according to the principles of the Declaration of Helsinki and was approved by the local ethics committees. The authors designed the study, gathered, and analyzed the data according to the STARD guidelines23,24 for studies of diagnostic accuracy (methods of the supplementary data), vouch for the data and analysis, drafted the paper, and decided to publish it.
Routine clinical assessmentAll patients underwent a clinical assessment that included medical history, physical examination, 12-lead ECG, continuous rhythm monitoring, pulse oximetry, standard blood test, and chest radiography, if indicated. Concentrations of cTn were measured at presentation and serially thereafter as long as clinically indicated. Treatment of patients, including drug therapy, was left to the discretion of the attending physician.
Adjudication of the final diagnosisIn all patients, adjudication of the final diagnosis was performed centrally in a core laboratory (Basel University Hospital). Two cardiologists reviewed all available medical records (patient history, physical examination, results of laboratory testing, radiologic testing, ECG, echocardiography, cardiac exercise test, lesion severity and morphology in coronary angiography, if available) pertaining to the patient from the time of ED presentation to the 90-day follow up. To take advantage of the higher sensitivity and higher overall diagnostic accuracy offered by hs-cTn,12,22,25 the adjudication was mainly based on serial hs-cTnT measured in a central laboratory from the serial study blood samples, additionally the adjudicating cardiologists had access to serial cTn/hs-cTn concentrations measured locally as part of routine clinical care. If there was disagreement about the diagnosis, the patients were reviewed and adjudicated in conjunction with a third cardiologist (approximately 10% of all patients).
AMI was defined and cTn concentrations interpreted using a uniform cutoff value (99th percentile) as recommended in current guidelines and as done in most contemporary large diagnostic studies.2,3,26 In brief, AMI was diagnosed when there was evidence of myocardial necrosis in association with a clinical setting consistent with myocardial ischemia. Myocardial necrosis was diagnosed by at least 1 hs-cTnT value above the uniform 99th percentile, 14 ng/L (for women and men) together with a significant rise and/or fall.3 Absolute changes in hs-cTnT were used to determine significant changes based on the diagnostic superiority of absolute over relative changes.21,27 Based on studies of the biological variation of cTn28 as well as on data from previous chest pain cohort studies,29,30 a significant absolute change was defined as a rise or fall of at least 10 ng/L within 6hours or 6 ng/L within 3hours.5,21,22
Patients with AMI were further subdivided into type 1 AMI (primary coronary events) and type 2 AMI (ischemia due to increased demand or decreased supply, for example tachyarrhythmia or hypertensive crisis).2,30
Measurement of high-sensitivity cardiac troponinIn all centers, blood samples for determination of the 3 hs-cTnT/I assays [hs-cTnT (Elecsys, Roche), hs-cTnI (Architect, Abbott), hs-cTnI (Centaur, Siemens)] were collected at presentation to the ED, after 1 hour, after 2hours and after 3hours in serum or plasma tubes during the recruitment period (from April 2006 to August 2015). Serial sampling was discontinued when the diagnosis was clear and required transfer, eg, to the catheter laboratory or coronary care unit. In addition, serial sampling had to be interrupted at the time of other diagnostic procedures, which required patient transfer to a different unit within the hospital, eg for computed tomography scans. After centrifugation, samples were frozen at −80°C until assayed in a blinded fashion in a dedicated core laboratory. Analytical details of the 3 hs-cTnT/I assays are described in the supplementary data.
Primary and secondary endpointsThe primary objective was to evaluate the possible near linearity of the acute release of hs-cTnT and hs-cTnI from cardiomyocytes into the circulation as quantified by acute changes of hs-cTnT and hs-cTnI concentrations in patients with early AMI. Changes from presentation to 1 hour (delta 0- to 1-hour) were compared with changes from presentation to 2hours (delta 0- to 2-hours) with 3 hs-cTnT/I assays.
The secondary objective was to compare the deltas 0- to 1-hour vs 0- to 3-hours to extend the observation to the first 3hours in the ED. Predefined subgroups included patients with non–ST-elevation acute myocardial infarction (NSTEMI), as the clinical implications of the linearity might be most profound in this population, type 1 AMI, type 2 AMI, patients presenting very early (within the first 2hours) after chest pain onset, as the release of hs-cTnT and hs-cTnI may be delayed for some time. Another subgroup included patients with total or subtotal coronary occlusion (culprit lesion severity 95% to 100%), as severely impaired (or even absent) coronary artery blood flow to the necrotic cardiomyocytes may affect release kinetics. The identification of the culprit lesion was left to the discretion of the attending physician.
Statistical analysisTo assess linearity, we performed linear regressions evaluating the correlation between both deltas (delta 0- to 1-hour: changes from presentation to 1 hour; vs delta 0- to 2-hours: changes from presentation to 2hours) with the correlation coefficients and slopes. Alternative analyses excluding outliers were also performed, showing no significant difference. A supplementary slope analysis was conducted to confirm near linearity: values at time points 1 and 2 hour were normed to the values at time point 0hours, and the linear regression slopes between 0- to 1-hour and 1- to 2-hours were plotted together and graphically compared with the linear regression slope between 0- and 2-hours. No formal sample size calculation was performed. A minimum a sample size within all assays was not predetermined because the main goal was to show real-world data for the implementation and monitoring of hs-cTn in a dynamic cohort and to provide a consistent internal validity within different assays.
Continuous variables are presented as median with interquartile ranges [IQR], categorical variables as numbers and percentages. All hypothesis testing was 2-tailed and P-values < .05 were considered statistically significant. All statistical analyses were performed using SPSS for Windows 25.0 (SPSS Inc, United States) and MedCalc 9.6.4.0 (MedCalc software, Belgium).
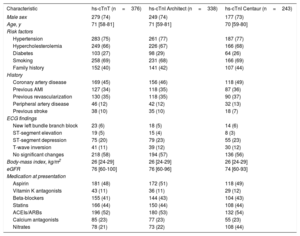
RESULTSPatient characteristicsPatient flow is shown in figure 1 of the supplementary data. Overall, 2437 patients had hs-cTnT concentrations available at presentation, after 1 hour and after 2hours, while 376 had a final diagnosis of AMI. Baseline characteristics were similar among the cohorts underlying the analyses of the 3 different hs-cTn assays (table 1): median age was 62 years, about one third of patients were women and about one third had known coronary artery disease.
Baseline characteristics
| Characteristic | hs-cTnT (n=376) | hs-cTnI Architect (n=338) | hs-cTnI Centaur (n=243) |
|---|---|---|---|
| Male sex | 279 (74) | 249 (74) | 177 (73) |
| Age, y | 71 [58-81] | 71 [59-81] | 70 [59-80] |
| Risk factors | |||
| Hypertension | 283 (75) | 261 (77) | 187 (77) |
| Hypercholesterolemia | 249 (66) | 226 (67) | 166 (68) |
| Diabetes | 103 (27) | 98 (29) | 64 (26) |
| Smoking | 258 (69) | 231 (68) | 166 (69) |
| Family history | 152 (40) | 141 (42) | 107 (44) |
| History | |||
| Coronary artery disease | 169 (45) | 156 (46) | 118 (49) |
| Previous AMI | 127 (34) | 118 (35) | 87 (36) |
| Previous revascularization | 130 (35) | 118 (35) | 90 (37) |
| Peripheral artery disease | 46 (12) | 42 (12) | 32 (13) |
| Previous stroke | 38 (10) | 35 (10) | 18 (7) |
| ECG findings | |||
| New left bundle branch block | 23 (6) | 18 (5) | 14 (6) |
| ST-segment elevation | 19 (5) | 15 (4) | 8 (3) |
| ST-segment depression | 75 (20) | 79 (23) | 55 (23) |
| T-wave inversion | 41 (11) | 39 (12) | 30 (12) |
| No significant changes | 218 (58) | 194 (57) | 136 (56) |
| Body-mass index, kg/m2 | 26 [24-29] | 26 [24-29] | 26 [24-29] |
| eGFR | 76 [60-100] | 76 [60-96] | 74 [60-93] |
| Medication at presentation | |||
| Aspirin | 181 (48) | 172 (51) | 118 (49) |
| Vitamin K antagonists | 43 (11) | 36 (11) | 29 (12) |
| Beta-blockers | 155 (41) | 144 (43) | 104 (43) |
| Statins | 166 (44) | 150 (44) | 108 (44) |
| ACEIs/ARBs | 196 (52) | 180 (53) | 132 (54) |
| Calcium antagonists | 85 (23) | 77 (23) | 55 (23) |
| Nitrates | 78 (21) | 73 (22) | 108 (44) |
ACEI, angiotensin converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; hs-cTn indicates high-sensitivity cardiac troponin.
Data are expressed as No. (%) or median [interquartile range].
The adjudicated final diagnosis was AMI in 15% of patients in the analysis of hs-cTnT, hs-cTnI Architect, and hs-cTnI Centaur. As shown in figure 1, among all 3 assays changes between 0 to 1 hour correlated strongly with changes between 0 to 2hours with correlation coefficients ranging from 0.931 to 0.969 (P <.001 for all assays). Additionally, figure 2 shows supplementary slope analysis. For each assay, the slopes 0 to 1 hour and 1 to 2hours were comparable with the slopes for 0 to 2hours, as given by overlapping confidence intervals of the linear regressions.
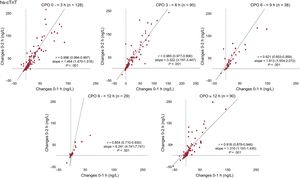
Findings in patients with NSTEMI were comparable to the overall AMI cohort (figure 3) with correlation coefficients ranging from 0.862 to 0.966 (P < .001 for all assays). Similarly, findings in patients with type 1 AMI were comparable to type 2 (results of the supplementary data, figure 2 of the supplementary data and figure 3 of the supplementary data). Results were comparable among all patients irrespective of the time since chest pain onset (figure 4, results of the supplementary data, figure 4 of the supplementary data) Correlations between 0- to 1-hour changes and 0- to 3-hour changes were similar to the correlations observed between 0- to 1-hour changes and 0- to 2-hour changes (results of the supplementary data, figure 5 of the supplementary data).
Correlation between changes at 0 to 1 hour and 0 to 2hours in AMI patients according to chest pain onset. Scatter plots showing the association between Delta 0- to 1-hour and 0- to 2-hour hs-cTnT among AMI patients according to time since chest pain onset in AMI patients. CPO, chest pain onset; hs-cTn, high-sensitivity cardiac troponin.
In the hs-cTnT dataset, coronary angiography was performed in 281 AMI patients. Among these patients, 27% had total coronary occlusion (100% diameter stenosis), 61% had a severe coronary culprit lesion stenosis (75%-99% diameter stenosis) and 12% had a less severe coronary culprit stenosis (< 75% diameter stenosis). The correlation between changes between 0 and 1hours and changes between 0 and 2hours was similar among all patients independently of the stenosis severity (figure 5). Similar results were observed for the 2 hs-cTnI assays (results of the supplementary data, figure 6 of the supplementary data).
Correlation between changes at 0 to 1 hour and 0 to 2hours in AMI patients according to stenosis grade. Scatter plots showing the association between Delta 0- to 1-hour and 0- to 2-hour hs-cTnT among AMI patients according to stenosis grade of the culprit lesion. Hs-cTn, high-sensitivity cardiac troponin.
This study was performed to contribute to a better understanding of the acute release kinetics of hs-cTnT and hs-cTnI from cardiomyocytes into the circulation within the first few hours of spontaneous AMI. It was our hypothesis that hs-cTnT and hs-cTnI release is near linear in these patients. We report 2 major findings.
First, in patients with AMI (including NSTEMI and ST-segment elevation myocardial infarction [STEMI]) acute 0- to 1-hour changes correlated very closely with 0- to 2-hour changes and with 0- to 3-hour changes. Additional slope analysis, where slopes 0 to 1 hour and 1 to 2 hour were comparable with the slopes 0 to 2 hour, confirmed the close correlations. These findings were highly consistent with all 3 hs-cTn assays and confirmed the hypothesis of a near linear release of hs-cTnT and hs-cTnI within the first few hours of AMI.
Second, release of hs-cTnT and hs-cTnI was also near linear also in all predefined subgroups including NSTEMI, type 1 and type 2 AMI, patients presenting very early after symptom onset, and patients with total/subtotal coronary occlusion.
These findings extend and corroborate previous observations, particularly those made from several experimental models that at least partly reflect the pathophysiology of spontaneous AMI in humans.11,31,32 After transcoronary alcohol ablation of septal hypertrophy, a combination of toxic and ischemic damage induced by temporary septal branch occlusion for selective therapeutic injection of 96% ethanol in 21 patients with hypertrophic obstructive cardiomyopathy, hs-cTnT plasma concentrations showed a near-linear increase.11 Assessing graded duration of acute coronary ischemia with ST depression vs release of cTnI using a conventional assay in 15 ischemic pigs, cTnI increased from 0.05 ug/L to 0.52 ug/L and 0.76 ug/L (P < .05) with 10 and 20minutes of ischemia, and to 30.77 ug/L (P <.05) with 30minutes of ischemia.32 In 452 patients with NSTEMI, hs-cTnT plasma concentrations showed a near linear increase with increasing time from symptom onset within the first few hours after arrival to the ED and then they plateaued.10
Recently, several distinct cellular mechanisms other than necrosis have been described to possibly result in hs-cTnT and hs-cTnI release from injured cardiomyocytes.33 These include apoptosis, transient increases in cell permeability due to cell wounds, formation and the release from membranous blebs or microparticles.33 In addition, experimental evidence suggests that first, cellular homeostasis may require active transport of hs-cTnT and hs-cTnI back into the cardiomyocytes, and second, that clearance mechanisms might differ between hs-cTnT and hs-cTnI.34 Putting these observations into perspective with our findings of consistent release kinetics among different AMI phenotypes, time intervals and different coronary lesion morphology, and among 3 different hs-cTn assays, it seems appropriated to conclude that the dominant mechanism seems to be identical for hs-cTnT and hs-cTnI in early AMI. These insights into AMI pathophysiology may help to advance our understanding of the temporal relationship between symptom onset and the development of cardiomyocyte necrosis. They seem to support the expansion of the “time is muscle” concept from the treatment of patients with STEMI to patients with NSTEMI. This has fundamental consequences and implies the need for very early and accurate diagnosis. Recently developed rapid hs-cTn-algorithms12–17 will have a major role in this. In addition, these novel insights into AMI pathophysiology might also help in the clinical implementation and adoption of rapid hs-cTn-algorithms, which use hs-cTn changes occurring within the first hour after ED presentation as surrogates for hs-cTn changes occurring within 3 or 6hours.12–15,17
Of note, our findings apply to early AMI, but not to the late phase of AMI, eg, 12 to 48hours after symptom onset, as blood concentrations of hs-cTnT and hs-cTnI plateau in the late phase of AMI and then fall again.10,11 While the vast majority of patients with AMI present within the first 12hours after chest pain onset, the inclusion criteria of this study, clinicians must appreciate the fundamental differences in release kinetics in late presenters. In these, concentrations at presentation are usually already markedly elevated, but do not show a relevant short-term change during serial sampling in the ED.
LimitationsThis study has some limitations. First, this study analyzed the near linearity of the acute release of hs-cTn using 3 hs-cTn assays. As the findings were consistent among the 3 different assays, we assume that they can be generalized to hs-cTnT/I in general but this assumption needs to be confirmed in additional studies. Second, we cannot comment on release kinetics in patients with end-stage kidney failure on chronic dialysis, since these patients were excluded from our study. Third, as a cohort of patients presenting with suspected AMI to the ED, our dataset underrepresents patients with STEMI, as these patients often are identified by the 12-lead ECG in the ambulance and taken directly to the cardiac catheterization laboratory, bypassing the ED. Therefore, the number of STEMIs was low in this cohort. Fourth, we could not assess release kinetics in patients in whom serial sampling was discontinued, because their diagnosis was clear and/or they required early transfer, eg to the cardiac catheterizastion laboratory or coronary care unit. As the diagnosis was clear at an even earlier time point in these patients, it is unlikely that release kinetics would have been different than those observed in this study. Fifth, these analyses are specific to patients presenting within 12hours after symptom onset or peak. While most patients with AMI present within this time window, late presenters represent an important minority. Usually, these patients have significant elevations in hs-cTn already at the first blood sampling, helping their identification as having AMI. Sixth, the documented near linearity of hs-cTnT and hs-cTnI release in early AMI does not allow us to delineate necrosis as the exclusive mechanisms involved. Seventh, since the results are based on an observational design, they only provide empirical evidence of the near linear release of hs-cTn in the first hours after myocardial ischemia, but they do not allow us to demonstrate the causal nature of this fact. Eighth, contrarily to the adjudication of final diagnoses, the interpretation of coronary angiograms and hence of the stenosis grade was performed by the treating physician and not centrally.
CONCLUSIONSPatients presenting with suspected AMI to the ED show a near linear release of hs-cTnT and hs-cTnI. Given the consistency of these findings among different AMI phenotypes, time intervals and coronary lesion morphology, and among 3 different hs-cTn assays, these results provide empirical evidence that the near linearity of release in early AMI applies to hs-cTnT and hs-cTnI in general. This near linearity provides the pathophysiological basis for rapid diagnostic algorithms and support for extending the concept of “time is muscle” to NSTEMI.
FUNDINGThis study was supported by research grants from the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the European Union, the University of Basel, Abbott, Beckman Coulter, Biomerieux, BRAHMS, Ortho Diagnostics, Roche, Siemens, Singulex, and the University Hospital Basel.
CONFLICTS OF INTERESTM. Rubini Giménez has received research grants from the Swiss Heart Foundation and Swiss National Science Foundation (P400PM_180828) as well as speakers honoraria from Roche, Abbott and Siemens. R. Twerenbold reports receiving research support from the Swiss National Science Foundation (P300PB-167803/1) and speaker honoraria/consulting honoraria from Roche, Abbott and Brahms. C. Mueller has received research support from the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University of Basel, the European Union, Abbott, Astra Zeneca, Beckman Coulter, Biomerieux, BRAHMS, Ortho Diagnostics, Roche, Siemens, Singulex, Sphingotec and the University Hospital Basel, as well as speaker honoraria and/or travel support from Abbott, Amgen, Astra Zeneca, Bayer, BG Medicine, Biomerieux, BMS, Boehringer Ingelheim, BRAHMS, Daiichi Sankyo, Novartis, Roche, Sanofi, Siemens, and Singulex. We disclose that T. Reichlin has received research grants from the Swiss National Science Foundation (PASMP3-136995), the Swiss Heart Foundation, the University of Basel, the Professor Max Cloetta Foundation and the Department of Internal Medicine, University Hospital Basel as well as speakers honoraria from Brahms and Roche. All other authors declare that they have no conflict of interest with this study. The sponsors had no role in the design of the study, the analysis of the data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
- -
The introduction of hs-cTn and its analytical superiority translated into clinical superiority in the early diagnosis of AMI.
- -
Insights into the release kinetics of cardiac troponins from cardiomyocytes into the circulation within the first hours of AMI are needed to advance our understanding of AMI pathophysiology including the temporal relationship between symptom onset and the development of cardiomyocyte necrosis and possible also help in the clinical implementation and adoption of recently developed rapid hs-cTn-algorithms.
- -
Based on our data, patients presenting with suspected AMI to the emergency department show a near-linear release of cardiac troponin T and I. This near-linearity provides the pathophysiological basis for rapid diagnostic algorithms.
We thank the patients who participated in the study, the staff of the emergency department, the research coordinators, and the laboratory technicians for their most valuable efforts.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.04.008