Keywords
INTRODUCTION
Conventional balloon stent implantation after predilatation is the standard treatment for most patients with coronary artery stenosis. Stent implantation without predilatation has become commonplace as a consequence of improved attachment of stents to balloons, reduced delivery profile and greater stent flexibility. The advantages of this technique are reductions in procedure time, exposure to radiation and cost.1,2 In addition, myocardial ischemia may be reduced as balloon inflation is needed on fewer occasions. Several authors have shown the procedure is feasible, effective and safe in selected patients.1-12 Angiographic follow-up studies have shown 11%-16% restenosis.13,14 Intravascular ultrasound (IVUS) studies after conventional stent angioplasty with predilatation have shown neointimal tissue proliferation is the principal mechanism in in-stent restenosis.15-17 However, the degree and pattern of neointimal tissue proliferation after direct stenting has hardly been studied. The present study was designed to use IVUS to analyze degree and axial distribution of neointimal tissue proliferation after direct stenting and identify variables that might predict this.
MATERIAL AND METHODS
Patients
We enrolled 50 patients with coronary artery anatomy and lesion morphology favorable for direct stenting. All presented clinical symptoms of angina with objective signs of myocardial ischemia. We excluded patients with calcified lesions, long lesions (>20 mm), total occlusions, extremely tortuous lesions in the segment proximal to the lesion and those with excessively angled vessels in the target segment. Presence of intraluminal thrombosis and clinical presentation of the illness were not selection criteria. In 5 patients (10%), we were unable to pass the stent through coronary artery stenosis and they were excluded from the study. Underestimating calcium presence in the lesion and attempting to cross very severe stenosis prevented direct stenting in these patients. The remaining 45 patients had successful direct stent implantations and underwent follow-up coronary angiography and IVUS studies at 7.85±2.81 months. In 23 patients potentially eligible for direct stenting, we performed conventional stent angioplasty with predilatation. In all of these patients we were able to cross the stent through the lesion after predilatation, so this was not the cause of exclusion for any of them.
Coronary Angioplasty
All patients received double oral antiplatelet treatment and 10 000 U intravenous sodium heparin prior to angioplasty. After femoral artery canalization using the Seldinger technique and locating a guide catheter in the coronary ostium we administered a 0.2-0.4 mg intracoronary bolus injection of nitroglycerin. Angiographic studies from at least 2 orthogonal projections were obtained. The standard technique of coronary stent implantation has been described elsewhere.18,19 In most patients undergoing implantation without predilatation, we used a high support guide wire. After passing the guide wire through the lesion we administered a further 0.2-0.4 mg intracoronary bolus injection of nitroglycerin to reverse possible associated vasoconstriction and attain maximum dilatation of the vessel. A stent/diameter ratio of 1.1-1.0/1.0 was used as reference. After stent deployment in the lesion, the balloon was gently inflated to above nominal pressure to ensure adequate expansion. If expansion was incomplete, balloon pressure was increased or a larger diameter balloon was used. This occurred in 4 direct stent patients but did not occur in any of the patients with predilatation (P=.35). When direct stenting was impossible, we used the same stent with previous predilatation and a conventional balloon. Patients stented without predilatation received Multi-Link stents (Guidant Corporation, Santa Clara, California) (20 patients), Jo-stent models (Jomed International AB, Rangendingen, Alemania) (20 patients), Tenax (Biotronik Gmbh & Co, Berlin, Germany), or NIR (Boston Scientific, Maple Grove, Minnesota) stents (5 patients). Patients undergoing implantation with predilatation received Multi-Link stents (17 patients) and NIR or Jo-stents (6 patients).
After angioplasty, we administered double antiplatelet treatment of nitrates and calcium antagonists for ≥1 month until the follow-up examination at least and acetylsalicylic acid indefinitely.
Angiographic Evaluation
Two observers analyzed the angiographic images. We obtained at least 2 orthogonal projections for analysis. We performed quantitative analysis using the border analysis system (Integris HM 3000, Philips Medical System, Leiden, Holland). Angiographic measurements were made in diastole after intracoronary administration of nitroglycerine and using the catheter guide wire for calibration. Percentage of stenosis, minimum luminal diameter (MLD) and lesion length were measured at baseline, immediately after stent implantation and at follow-up. The variables calculated were: 1) acute gain, defined as increased target artery MLD after stent implantation; 2) late loss, defined as reduction in target artery luminal diameter in angiographic follow-up; and 3) net gain, defined as the difference between acute gain and late loss.20
Intracoronary Echography
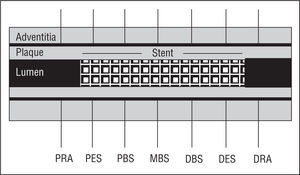
Our equipment consisted of a 30-MHz transducer mounted at the acoustic tip of a 3.2 F catheter (CVIS Inc., Sunnyvale, California) connected to an ultrasound console. After administering 5000 U heparin and 0.2-0.4 mg intracoronary nitroglycerine, the intravascular imaging system was passed through a 0.014 guide wire to the distal reference segment, 3-4 mm distal to the stent edge. We performed IVUS studies, withdrawing the intravascular imaging system at 0.5 mm/s until reaching the proximal reference diameter, 3-4 mm proximal to the stent edge. Ultrasound images were recorded on video for later analysis. We made seven quantitative measurements of which 5 were in the stented segment: the proximal edge of the stent, the proximal body of the stent, the mid-body of the stent, the distal body of the stent and the distal edge of the stent; and 2 in the non-stented proximal and distal segments (Figure 1).
Figure 1. Schema of artery and reference segments determined by intravascular echography. PRA indicates proximal reference area; PES, proximal edge of the stent; PBS, proximal body of the stent; MBS, mid-body of the stent; DBS, distal body of the stent; DES, distal edge of the stent; DRA: distal reference area.
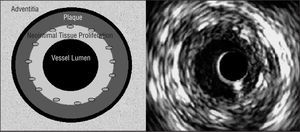
Cross-sectional area (CSA) measurements in the stented coronary artery segment: 1) vessel CSA, obtained by tracing the media-adventitia edge (equivalent to the external elastic membrane); 2) stent CSA, measuring stent circumference; and 3) vessel luminal CSA, obtained by tracing the luminal edge of the area of neointimal tissue proliferation (Figure 2). The percentage of plaque extruded out of the stent was calculated by subtracting vessel CSA from stent CSA. The result, the area of plaque extruded (plus the media) out of the stent, was divided by vessel CSA and multiplied by 100. We calculated vessel CSA and luminal CSA in the non-stented part of the coronary artery. The degree of neointimal tissue proliferation in each of the 5 stent cross-sections was calculated as follows: stent CSA minus luminal CSA, divided by stent CSA, multiplied by 100.
Figure 2. Left: schema of cross-sectional areas determined by intravascular echography in the stented segment. We measured the area of the vessel (on the adventitial edge of the external elastic membrane), and the areas of the stent and the vessel lumen. We calculated the percentage of plaque extruded out of the stent, including the media (represented as a black circumference between the plaque and the adventitia) and intimal proliferation. Right: intracoronary echography image. The circumference in the interior of the lumen corresponds to the catheter (not represented in the schema on the left).
Statistical Analysis
We tabulated angiographic results at baseline, procedure and post-stenting, and late diameters at follow-up. Results of the IVUS study were analyzed at follow-up. To detect differences between continuous variables in patients with and patients without predilatation we used Student's t test and χ² for categorical variables. We used analysis of variance to detect differences in CSA measurements along the coronary artery stent axis (with stent and reference vessel). The mean of the 5 stent measurements was used to analyze correlations between continuous variables. Pearson's correlation coefficient was used to quantify linear relationships between continuous variables. Results are expressed as percentages and as mean ± 2 standard deviations. Statistical significance was set at P≤.05.
RESULTS
Clinical Results of Procedure and Angiography
All lesions analyzed were type A and B with a length of 11.5±4.5 mm. Baseline clinical and angiographic characteristics were comparable in the 2 groups (Table 1). Similarly, baseline, immediate post-procedure, and late data for MLD, percentage of stenosis, late loss, and net gain did not differ between the 2 groups (Table 2). However, analysis of procedural results revealed that greater inflation pressure was used in direct stenting than in conventional stent angioplasty with dilatation (13±3 atm vs 10±2 atm, P=.005). Inflation time, stent length, length of lesion/length of stent, and stent/reference artery diameter ratios showed no differences between the 2 groups.
IVUS and Follow-up Results
Overall Results
We evaluated 340 cross-sectional cuts of target coronary artery segments in 68 patients. Vessel CSA, stent CSA, and luminal CSA were 15.6±3.6 mm², 8.5±2.2 mm², and 5.9±2.0 mm², respectively. The percentage of plaque extruded from the stent was 44.8%±9.4% and neointimal tissue proliferation was 30.1%±14.9%. We analyzed 136 cross-sectional cuts in the reference artery segment. Proximal and distal vessel CSA were 16.7±3.9 mm² and 14.9±3.6 mm², respectively. Proximal and distal luminal CSA were 9.7±3.2 mm² and 8.5±2.7 mm², respectively.
Comparison of IVUS Results for the 2 Groups
Detailed analysis of CSA of the 5 stent cross-sections and reference artery segment CSA in the 2 groups is in Table 3. Vessel CSA and stent CSA were significantly greater in most of the cross-sections in the group without predilatation by comparison with the group with predilatation. We found no differences in vessel CSA and luminal CSA in the proximal and distal coronary artery reference segment between the 2 groups.
Neointimal tissue proliferation was slightly but non-significantly greater in the group without predilatation by comparison the group with predilatation (Table 3). The percentage of plaque extruded from the stent was slightly greater in the group with predilatation and the difference was statistically significant around the mid-body of the stent when comparing the 2 groups (51.1%±7.2% vs 46.4%±6.7%; P=.02).
Comparison of IVUS Results Along the Coronary Artery Axis
Target vessel CSA and distal reference artery CSA were less than proximal reference artery CSA in both groups (P<.05). Luminal CSA in the area of the stent in the 2 groups was less than luminal CSA of the proximal and distal reference artery (P<.05). The factor determining this narrowness was neointimal tissue proliferation (Table 3 and Figure 3).
Figure 3. Graphs of intravascular echography measurements of vessel, stent and lumen cross-sectional areas in patients with (left) and without (right) predilatation. The areas along the axis of the stented artery are similar in both groups. Cross-sectional area reduction in-stent is explained by neointimal tissue proliferation. We did not find a heterogeneous increase along the stent in the 2 groups. PRA indicates proximal reference area; PES, proximal edge of the stent; PBS, proximal body of the stent; MBS, mid-body of the stent; DBS, distal body of the stent; DES, distal edge of the stent; DRA, distal reference area; CSA, cross-sectional area; NS, non-significant. *P<.05 between proximal or distal reference CSA and coronary artery stented segment.
Stent CSA and degree of neointimal tissue proliferation in the 5 predetermined stent axis sections were similar in the 2 groups indicating homogeneous neointimal growth along the stent in the 2 groups.
Variables Predicting Neointimal Tissue Proliferation
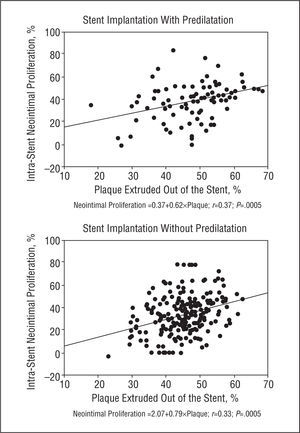
Minimum luminal diameter and percentage of stenosis did not correlate with neointimal tissue proliferation in baseline or immediate post-procedure angiography. However, we found a weak linear relationship between plaque outside the stent and neointimal tissue proliferation in the group with predilatation (r=0.37; P=.005) and the group without predilatation (r=0.33; P=.005) (Figure 4). Neointimal tissue proliferation did not correlate with inflation pressure or stent CSA as determined by IVUS.
Figure 4. Correlation between plaque extruded out of the stent and neointimal tissue proliferation in the group with (above) and without (below) predilatation. We describe the predictive equation in each group.
DISCUSSION
Several clinical studies have shown that coronary artery stent implantation reduces angiographic restenosis and need for reoperation when compared with conventional balloon angioplasty.18,19 Intravascular ultrasound studies have shown that after stent implantation neither shrinkage nor narrowing of the prosthesis occur in follow-up. In fact, diagnosis of late stent stenosis represents incomplete expansion of the stent not detected at the time of implantation.15 The IVUS studies have enabled us to show that late luminal reduction is due to neointimal tissue proliferation.16,17 This neointimal growth is uniformly distributed along the stent implanted with predilatation.17 In the Palma-Schatz stent we found a smaller luminal diameter in the central articulation. This is due to the combination of intimal tissue prolapse and neointimal tissue formation in this area. In the present series, we found homogenous distribution of neointimal tissue proliferation after direct stenting along the stent axis, as occurs in conventional stent angioplasty. We did not identify preferred foci of neointimal tissue proliferation along the prosthesis. Unlike previous IVUS studies in articulated stents,15-17 the use of tubular stents can explain our results. These stents provide rigid scaffolding for the arterial wall21,22 and uniform coverage of the lesion along the stent; and they prevent potential prolapse of the plaque through the prosthesis cells that might predispose abnormal neointimal growth.23 Although it has been suggested that direct stenting can reduce endothelial denudation provoking less neointimal tissue proliferation and a lower rate of restenosis,24 angiographic follow-up studies have shown a similar rate of restenosis with both techniques.13,14,25,26 In our series, angiography revealed late loss and IVUS showed slight similarities in intimal proliferation in patients with and without predilatation.
In the present study, analysis of stent CSA along the coronary artery segment was similar in the 2 groups. This suggests uniform deployment of the stent with both techniques. As most patients presented with acute coronary syndromes, it is highly likely that plaque was principally "soft," rather than "fibrous" or "calcified." This would facilitate spatial redistribution of plaque during implantation and uniform expansion of the stent. In the present series, angiographic calcification, associated with incomplete expansion, was an exclusion criterion. The above-normal inflation pressures used may also have contributed to uniform stent expansion. In direct stenting, we used greater inflation pressure than in conventional stent angioplasty (13±3 vs 10±2; P<.005). Stent CSA and luminal CSA were greater with direct stenting. As reference artery CSA and the artery diameter/reference diameter ratio are similar in the 2 groups, the parameter determining increase in these areas was probably greater inflation pressure during stent delivery. The implantation procedure would favor the rupture, distribution and crushing of plaque. Here, we found that the percentage of plaque extruded out of the stent was less in direct stenting than in stenting with predilatation. However, the greater stent area found with direct stenting may be partly due to greater incidence of "soft" plaque by comparison with stenting with predilatation because of the greater incidence of unstable angina in direct stenting (82.2% vs 65.2%; P=.2). This last characteristic, greater instability and possible plaque inflammation in the direct stent group, together with lower inflation pressure in the group of stenting with predilatation, are factors that could have influenced subsequent neointimal tissue proliferation and our results (note data on late loss in Table 2 and intimal proliferation in Table 3).
Increased stent CSA can cause a proportional increase of the prosthesis surface producing a parallel increase in neointimal tissue proliferation. This would explain the slight, non-significant increase of neointimal tissue proliferation observed following direct stenting by comparison with stenting with predilatation. Note that balloon inflation pressure used to implant the stent was not pre-established, reaching the pressure needed to obtain complete angiographic balloon expansion and an optimal immediate result. Conventional balloon predilatation causes fissures, lesser dissections, rupture, compression and plaque redistribution. This leads to widening of luminal CSA that facilitates stent implantation. On the other hand, direct stenting requires greater radial expansion force to break down and distribute the plaque spatially and permit uniform stent deployment. Increasing inflation pressure is probably a better means of achieving this. A recent IVUS study showed better angiographic and ultrasound results after direct stenting when higher inflation pressures are used for implantation.27
It has been reported that amount of plaque predicts restenosis following conventional balloon angioplasty and atherectomy.28 We concur with other authors29 who found a weak linear relationship between degree of neointimal tissue proliferation and percentage of plaque outside the stent. In the present study, we found that this relationship holds true for direct stenting.
Limitations of the Study
Patients were selected on criteria relating to coronary artery anatomy and morphology of favorable lesions. The cross-sectional design of the study precludes evaluation of pre-intervention variables related with restenosis. Other series using IVUS after stenting with predilatation found baseline plaque load and post-intervention luminal area can predict intra-stent restenosis.30 In the present study we did not evaluate stent shrinkage as previous studies show good angiographic results and good resistance to radial compression with new generation stents.21-23 Although our study design was not random, significant differences between baseline clinical and angiographic characteristics did not appear and this suggests the groups were homogeneous. Furthermore, it should be borne in mind that the sample may have been too small to detect differences between the groups, meaning comparisons must be interpreted with caution. These may also be conditioned by the fact that inflation pressures in the group with predilatation were lower than in the group without predilatation and lower than those normally used. On the other hand, 5 direct stent patients were excluded from the study as they presented very severe or moderately calcified coronary artery stenosis. This could explain our finding more plaque outside the stent mid-body in patients with predilatation by comparison with patients without predilatation. However, we did not find significant differences in amount of plaque along the stent axis.
In conclusion, as in conventional stent angioplasty, in selected patients undergoing direct stenting, a homogeneous proliferative neointimal response occurs along the longitudinal stent axis which correlates weakly with the amount of plaque extruded out of the stent.
Correspondence: Dr. V. Martí.
Unidad de Hemodinámica. Hospital de la Santa Creu i de Sant Pau.
Sant Antoni M. Claret, 167. 08025 Barcelona. España.
E-mail: vmc18461b@hotmail.com