Myocardial dysfunction, systolic or diastolic, of either the right or left ventricle, is a common final pathway in a number of congenital heart defects, often leading to clinical heart failure, arrhythmia, and death. Myocardial fibrosis, detectable with today's imaging techniques, likely plays a role in the gradual deterioration of myocardial function.1 This role has been most convincingly investigated and demonstrated in patients with tetralogy of Fallot (TOF) and, to a lesser degree, in those with a systemic right ventricle (RV) in the setting of transposition of the great arteries (TGA). The sum total of the evidence thus far provides conviction of the important role played by fibrosis in the natural history of these conditions. With imaging tools to detect and quantify fibrosis, we can potentially study the progression of myocardial changes over time, investigate factors that most heavily influence fibrogenesis, provide prognostication (eg, identify arrhythmia risk, congestive heart failure events), or even identify a potential surrogate endpoint for pharmacotherapy studies. Herein we review the current evidence for fibrosis, focusing on these 2 conditions, and its clinical associations.
The bulk of myocardial fibrosis imaging today is done via 2 commonly used techniques using cardiac magnetic resonance (CMR) and gadolinium contrast, and thus patients who are not candidates for CMR or contrast administration are not imaged. Both techniques are based on the concept that fibrosis increases the extracellular space. Since gadolinium contrast agents are strictly extracellular, their effects will be more prominent when the extracellular space is enlarged. Contrast shortens T1, in other words, it decreases the time required for spinning protons to realign with the longitudinal magnetization axis in the scanner following an excitation pulse that inverts the axis of those spinning protons. Thus the T1 time will differ in areas that are densely extracellular, including fibrosed areas, and this can be determined with imaging.
In late gadolinium enhancement (LGE), also referred to as delayed gadolinium enhancement, images are acquired roughly 10 to 30minutes after gadolinium contrast injection. Dense areas of myocardial extracellular space, which will retain more contrast than areas of normal myocardium, will therefore have a shorter T1 time and higher signal intensity than normal myocardium. When large enough, such areas can be detected visually as a focal plaque or scar. High signal areas, or “enhanced” areas, can be outlined and quantified, although this process can be somewhat subjective. Many congenital heart disease patients likely have more diffuse myocardial fibrosis, however, and LGE, which is largely a dichotomous method, may be less effective for either detection or quantification.2 For diffuse fibrosis, imaging can be done to quantify T1 values on a pixel-by-pixel basis. By measuring the T1 value of each pixel in the myocardium before and after administration of gadolinium, and plotting the change against that of the blood pool, one can calculate the volume of distribution of gadolinium within the myocardium, which can be converted to an extracellular volume fraction (ECV).1 ECV demonstrates diffuse fibrosis in adult congenital heart disease patients with various lesions, and correlates with ventricular size and function.3 ECV is largely objective, but requires careful contouring to avoid extracellular fat or myocardial trabeculations.
Both of these techniques have been used extensively across various cardiac conditions, repeatedly showing associations with other markers of adverse cardiac function or clinical status. In congenital heart disease, TOF and TGA have been most closely studied with these methods. It is important to recognize differences between these methods, as differences in the degree of fibrosis will depend on the method used, as will be discussed below. One key difference is that ECV, for now, covers a smaller sample of the myocardium than does LGE. Hence, LGE may be more sensitive for overall burden of fibrosis, even though it requires a greater amount to be detectable visually.
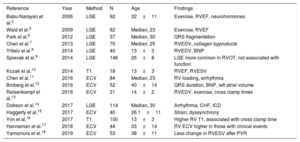
FIBROSIS IN TETRALOGY OF FALLOTIn TOF, the first study to show LGE after repair included 92 participants.4 Not unexpectedly, LGE was nearly universally found in the right ventricular outflow tract at or near a transannular patch, or ventricular septal defect patch. However, pathologic left ventricular LGE was also shown in 13% of participants. The degree of LGE present was associated with age, symptoms, prior arrhythmia, B-type natriuretic peptide (BNP), exercise capacity, and RV volume and function. Several studies have since followed, collectively showing various findings around this basic theme; more fibrosis associated with adverse clinical findings (table 1). LGE has been demonstrated in both the RV and LV, even though the RV is perceived to bear the brunt of adverse loading conditions. LV involvement is yet another confirmation that both ventricles are affected in TOF through various coupling mechanisms.
Studies on myocardial fibrosis in tetralogy of Fallot
| Reference | Year | Method | N | Age | Findings |
|---|---|---|---|---|---|
| Babu-Narayan et al.4 | 2006 | LGE | 92 | 32±11 | Exercise, RVEF, neurohormones |
| Wald et al.5 | 2009 | LGE | 62 | Median, 23 | Exercise, RVEF |
| Park et al.6 | 2012 | LGE | 37 | Median, 30 | QRS fragmentation |
| Chen et al.7 | 2013 | LGE | 70 | Median, 25 | RVEDV, collagen byproducts |
| Ylitalo et al.8 | 2014 | LGE | 40 | 13±3 | RVEDV, BNP |
| Spievak et al.9 | 2014 | LGE | 146 | 26±8 | LGE more common in RVOT, not associated with function |
| Kozak et al.10 | 2014 | T1 | 18 | 13±3 | RVEF, RVESV |
| Chen et al.11 | 2016 | ECV | 84 | Median, 23 | RV loading, arrhythmia |
| Broberg et al.12 | 2016 | ECV | 52 | 40±14 | QRS duration, BNP, left atrial volume |
| Reisenkampf et al.13 | 2016 | ECV | 31 | 14±2 | RVEDV, exercise, cross clamp times |
| Dobson et al.14 | 2017 | LGE | 114 | Median, 30 | Arrhythmia, CHF, ICD |
| Haggerty et al.15 | 2017 | ECV | 40 | 26.1±11 | Strain, dyssynchrony |
| Yim et al.16 | 2017 | T1 | 100 | 13±3 | Higher RV T1, associated with cross clamp time |
| Hanneman et al.17 | 2018 | ECV | 44 | 33±14 | RV ECV higher in those with clinical events |
| Yamamura et al.18 | 2019 | ECV | 53 | 38±11 | Less change in RVESV after PVR |
BNP, B-type natriuretic peptide; CHF, congestive heart failure; ECV, extracellular volume fraction; ICD, implantable cardioverter-defibrillator; LGE, late gadolinium enhancement; PVR, pulmonary valve replacement; RV, right ventricle; RVEDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RVESV, right ventricular end-systolic volume; RVOT, right ventricular outflow tract.
Unless stated otherwise, age is given as mean±standard deviation.
Newer studies have incorporated ECV for diffuse fibrosis imaging, and collectively demonstrate similar overall findings but with variation between studies. Investigators at Boston Children's hospital have shown increased RV and LV ECV in 84 TOF patients (mean age 24 years).11 Abnormal LV ECV values were found in 11 participants. A higher RV ECV was found in those with volume loading as opposed to pressure loading of the RV. Published simultaneously was a study in 52 older participants (mean age 40±14 years) and 22 controls, wherein LV ECV was higher in patients than controls (28.2±3.9% vs 26.1±2.0%; P=.026).12 RV ECV was not measured. Importantly, elevated LV ECV was associated with older age, age at TOF repair, higher NT-BNP, longer QRS duration, shorter 6-minute walk distance, and larger left atrial volume. In a separate study of younger participants (age 13.9±2.4 years), native T1 times (before contrast administration) and RV ECV was associated with longer bypass and aortic cross clamp times during initial repair, as well as peak exercise capacity.13,16 In another study in adults, 40 participants age 26±11 years demonstrated that LV ECV values were associated with LV dyssynchrony and radial strain.15
Hence these cumulative studies, covering a range of ages, though varying in some of their findings, each contribute to an emerging picture showing the role of RV and LV fibrosis in the natural history following repair. Additional confirmation comes from studies showing that clinical outcome is worse in those with demonstrable fibrosis. In one study in patients after pulmonary valve replacement, the degree of RV size reduction was lower in those with RV fibrosis.18 In another study of 52 adult participants, arrhythmia or congestive heart failure outcomes after 4 years were more prevalent in those with higher LV ECV values.12 In yet another, in 44 adult patients with a median follow up of only 8 months, RV ECV was associated with an endpoint of death, congestive heart failure, or VT.17 Though small, the generally consistent message across these studies is that fibrosis has an impact on outcome.
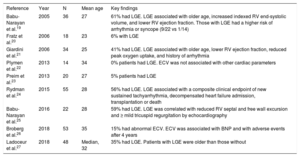
TRANSPOSITION OF THE GREAT ARTERIES WITH A SYSTEMIC RIGHT VENTRICLEIn the systemic RV of those with TGA, fibrosis has also been demonstrated using both LGE and ECV techniques. Existing studies are summarized (table 2), which, though limited, again collectively demonstrate that myocardial fibrosis is associated with clinical deterioration. Several of these are worth specific mention herein.
Summary of the current data regarding late gadolinium enhancement or extracellular volume fraction in the systemic right ventricle
| Reference | Year | N | Mean age | Key findings |
|---|---|---|---|---|
| Babu-Narayan et al.19 | 2005 | 36 | 27 | 61% had LGE. LGE associated with older age, increased indexed RV end-systolic volume, and lower RV ejection fraction. Those with LGE had a higher risk of arrhythmia or syncope (9/22 vs 1/14) |
| Fratz et al.20 | 2006 | 18 | 23 | 6% with LGE |
| Giardini et al.21 | 2006 | 34 | 25 | 41% had LGE. LGE associated with older age, lower RV ejection fraction, reduced peak oxygen uptake, and history of arrhythmia |
| Plymen et al.22 | 2013 | 14 | 34 | 0% patients had LGE. ECV was not associated with other cardiac parameters |
| Preim et al.23 | 2013 | 20 | 27 | 5% patients had LGE |
| Rydman et al.24 | 2015 | 55 | 28 | 56% had LGE. LGE associated with a composite clinical endpoint of new sustained tachyarrhythmia, decompensated heart failure admission, transplantation or death |
| Babu-Narayan et al.25 | 2016 | 22 | 28 | 59% had LGE. LGE was correlated with reduced RV septal and free wall excursion and ≥ mild tricuspid regurgitation by echocardiography |
| Broberg et al.26 | 2018 | 53 | 35 | 15% had abnormal ECV. ECV was associated with BNP and with adverse events after 4 years |
| Ladoceur et al.27 | 2018 | 48 | Median, 32 | 35% had LGE. Patients with LGE were older than those without |
BNP, B-type natriuretic peptide; ECV, extracellular volume fraction; LGE, late gadolinium enhancement; RV, right ventricle.
LGE has demonstrated systemic RV fibrosis in up to 61% of participants.19 Affected patients are older and have worse systemic RV function than unaffected patients. They also have more arrhythmia and syncope, adding credibility as a marker of myocardial damage.19 Others have reported similar results and associations,21 including with wall stress and collagen biomarkers.27,28 This later association importantly supports the hypothesis that LGE represents true myocardial fibrosis rather than other changes such as myocardial edema. LGE extent is associated with dyssynchrony in addition to lower exercise capacity and prior arrhythmia, implying a link between the fibrotic process, electromechanical delay, and clinical events.25
Like with TOF, the collective sum of available research strongly suggests that myocardial fibrosis plays a part in the natural history of the systemic RV failure, yet not all studies show a similar prevalence of LGE, and some show none.20,22,23 The discrepancy may reflect the subjective nature of LGE, especially among dense trabeculations. Alternatively, patient age or surgical techniques may vary, reflecting inherent heterogeneity. Additionally, most studies were done exclusively in D-TGA, excluding cc-TGA.
Only limited studies address whether detecting fibrosis can alter prognosis. The largest included 55 patients, 56% with LGE.24 RV LGE was independently associated with adverse outcome after 7.8 years (combined endpoint of sustained tachyarrhythmia, heart failure admission, transplantation, or death). In addition, there has been a clinical-pathologic correlation with an explanted heart after transplantation.2 Though this supports the idea that fibrosis is an important mediator of cardiovascular dysfunction and clinical events, there are still questions whether the presence of LGE should direct patient care.
The first focused study of ECV in the systemic RV was done on 14 D-TGA patients after the atrial switch using an equilibrium contrast method during a constant infusion of gadolinium.22 Interestingly, no focal LGE was seen in any patient. Septal ECV was higher than in controls, but ECV of the RV free wall was unmeasurable. There was no correlation between ECV and RV volume or ejection fraction.
A larger prospective study investigated 53 patients (both D- and congenitally corrected TGA varieties) and controls.26 The RV ECV was abnormal in 29%. Patients with an elevated ECV did not differ significantly compared with those with normal ECVs, and there was no difference in RV volume, RV ejection fraction, tricuspid regurgitation severity, arrhythmia history, medication use, circulating collagen byproducts, or aldosterone, but BNP levels were higher. After a median follow up of 4.4 years, those with an elevated ECV had more frequent heart failure events than those without, despite the lack of association with other clinical signs of heart failure. While it is unwise to speculate from one small study, the results support the hypothesis that fibrosis plays a strong role in the development of clinical heart failure.
Some shortcomings of ECV must be highlighted. RV ECV quantification can be more laborious than LV. It is complicated by potential trabecular contrast pooling. The erroneous inclusion of pooling lowers postcontrast T1 values and increases ECV. Some LGE studies suggest fibrosis is more prevalent than by ECV, when the latter should be more sensitive. This paradoxical difference may in part relate to sampling; T1 acquisitions are usually limited to only 1 to 3 short axis planes rather than the entirety of the ventricle as is done for LGE. Also, since ECV is a continuous measure, small amounts of fibrosis may still result in a “normal” ECV even though the fibrotic process is already present. Cutoffs for normal values may differ between laboratories, and the variance between measurements over time is not well established. It is also unclear whether differences in strain correlate with fibrosis.
Enthusiasm for the widespread clinical use of ECV has been lessened by the fact that clinical management is not yet informed by results. Though the link between fibrosis and ventricular dysfunction appears undeniable, pharmacotherapies to attenuate fibrosis have not necessarily led to clinical improvement, and decisions about implantable cardioverter-defibrillator use are not informed by ECV.
CONCLUSIONSA major imaging goal in congenital heart disease is to assess myocardial volume and function. However, it is also to characterize important changes in the myocardium itself that reflect underlying pathologic changes in response to adverse loading. This includes the presence of myocardial fibrosis. LGE and ECV studies in both TOF and TGA collectively paint a picture of myocardial fibrosis as a linchpin in the gradual deterioration of ventricular function and adverse clinical outcomes. While management decisions based on some of these new applications are still empiric, fibrosis detection could theoretically serve as an important surrogate endpoint for future pharmacologic studies, or inform future decisions about various therapy options.
Conflicts of InterestNone declared.