Keywords
Adhesion receptors play an essential physiological role in maintaining tissue integrity by regulating numerous processes such as cell activation, migration, growth, differentiation, and death.1,2 This regulation is achieved through direct signal transduction and modulation of intracellular signaling triggered by different growth factors.3 Cell-cell interactions are essential for regulating hematopoiesis4,5 and the inflammatory response.6,7 Adhesion molecules are therefore particularly implicated in a wide variety of cardiovascular disorders that involve inflammation, such as atherogenic processes and progression of atherosclerotic plaque, myocardial infarction, ischemia-reperfusion injury or transplant rejection, and, to a lesser extent, valve stenosis, and myocardiopathy.
The coordinated functioning of adhesion receptors, the cytoskeleton, and signaling molecules is crucial for leukocyte extravasation, a key process in immune response. Thus, correct integration of signals from "outside in" and "inside out" in leukocytes and the endothelium during each extravasation step—the so-called multi-step paradigm—is essential for this phenomenon to occur6,8 (Figure 1). Leukocyte extravasation takes place not only during inflammatory response, but also during recirculation of the lymphocytes to the secondary lymphoid organs, although this latter process will not be considered in this review.
Figure. 1. The adhesion cascade. The scanning electron microscope image shows a human endothelial monolayer treated with proinflammatory stimuli and perfused with human peripheral blood lymphocytes and monocytes at physiological flow (1.8 dyn/cm2). Several unpolarized leukocytes have come into contact with the endothelium and have been captured during the rolling process. Also shown is a lymphocyte that has managed to firmly adhere to the endothelium and drastically changed its morphology from rounded to polarized.
INITIAL INTERACTIONS BETWEEN CIRCULATING LEUKOCYTES AND THE ENDOTHELIUM: TETHERING AND ROLLING MEDIATED BY SELECTINS AND THEIR LIGANDS
To initiate the inflammatory response, circulating leukocytes in the bloodstream have to establish contact (tethering) with the vascular wall and adhere to it, while withstanding the shear forces. Tethering and rolling of the leukocytes over the activated endothelium are the first steps in the sequential process of extravasation. They are followed by firm adhesion and transendothelial migration. The initial contact or tethering is largely mediated by selectins and their ligands, and blood flow must be present for it to be efficient.9 Although selectins and their ligands tend to interact with a variable affinity, the high frequency of association-dissociation of interactions allows them to mediate labile and transient tethers between leukocytes and the endothelium.10,11 Tethering slows the speed of travel of the leukocytes and allows them to roll over the endothelial surface, favoring subsequent interactions mediated by integrins and their ligands and increasing leukocyte adherence. As a result, the leukocytes finally come to a halt on the vascular wall.12
The selectins (P, E, and L) are type 1 transmembrane glycoproteins that bind to fucosylated and sialylated hydrocarbons present in their ligands in a Ca2+-dependent fashion. L-selectin is expressed by most leukocytes, whereas the E and P forms are expressed on endothelial cells activated by proinflammatory stimuli. P-selectin is also expressed by activated platelets (reviewed by Barreiro et al13). In addition to the interaction of leukocyte selectin (L-selectin) with endothelial selectin (P- and E-selectin), the P-Selectin glycoprotein ligand-1 (PSGL1) protein is a major ligand of these 3 selectins. In fact, the binding of PSGL1 to P- and E-selectin promotes the interaction of leukocytes with the endothelium, whereas the binding of PSGL1 to L-selectin enables leukocyte-leukocyte interactions, whereby the adhered leukocytes facilitate the capture of other circulating leukocytes at sites where the endothelium is inflamed, regardless of whether these cells express ligands for endothelial selectins in a process denoted secondary recruitment.14 In addition to PSGL1, selectins can also bind to other glycoproteins such as CD44 or E-selectinligand-1 (ESL1) in the case of E-selectin. Each particular ligand seems to have a distinct function during the process of neutrophil capture. Thus, PSGL1 is primarily implicated in initial leukocyte tethering, whereas ESL1 is necessary to convert the transient initial tethers into a slower and more stable rolling. Finally, CD44 controls the rolling velocity and intervenes in the polarization of PSGL1 and L-selectin, probably to allow secondary recruitment.15 Platelets can also act as secondary recruiters of leukocytes thanks to their capacity to interact with both the circulating leukocytes and the endothelium at the same time. In addition, they can release chemokines that are immobilized on the luminal endothelial surface, thereby favoring the adhesion process.16
Besides the selectins and their ligands, the α4β1-andα4β7-integrins—through their interaction with vascular cell adhesion molecule (VCAM) 1 and mucosal addressin cell adhesion molecule (MAdCAM) 1, respectively—can independently mediate this initial tethering.17-19 On the other hand, the interaction between lymphocyte function-associated antigen (LFA) 1 and cell-cell adhesion molecule (ICAM) 1 collaborates with the function of L-selectin, thereby stabilizing the transient contact phase and reducing the rolling velocity.20,21
The adhesion receptors must be correctly localized on the cells for them to function correctly during leukocyte trafficking.22 Selectins and their ligands, and the α4 integrins are clustered at the ends of the microvilli of the leukocytes. The tethering of the selectins to the cytoskeleton of actin with proteins such as α-actinin or ezrin/radixin/ moesin (ERM) is necessary for them to function properly.23-26
It has been shown that selectins activate multiple signaling pathways that are linked to processes such as actin cytoskeletal reorganization, like the MAPK, p56lck, Ras, or Rac2 cascade (reviewed by Barreiro et al13). On the other hand, PSGL1 also activates different intracellular signaling pathways with an inductive effect on the activation of leukocytes, thereby increasing the expression of different molecules that are implicated in the following steps of the extravasation process and in effector function. They also play an unexpected role in the induction of tolerogenic function in dendritic cells.27-30
CENTRAL ROLE OF LEUKOCYTE INTEGRINS AND THEIR ENDOTHELIAL LIGANDS IN PROCESSES OF ACTIVATION, ARREST, FIRM ADHESION, AND CRAWLING
Leukocyte trafficking through the different tissues and organs and the subsequent leukocyte interaction with other immune cells are essential for developing innate and acquired immunity.31 Integrins are fundamental molecules in cell migration. They control the cell-cell and cell-extracellular matrix interactions during recirculation and inflammation. One of their most important characteristics lies in their ability to alter their adherent activity, regardless of how extensively expressed they are on the membrane.32 Thus, circulating leukocytes in blood maintain their integrins in an inactive conformation to avoid nonspecific contact with uninflamed vascular walls, but when they arrive at the inflammatory focus, a rapid in situ activation of the integrins occurs.33 As in the case of the selectins, the spatial distribution of the integrins and their ligands on specialized membrane structures is essential for proper function. This spatial organization requires a precise regulation of the cytoskeleton to allow recruitment of signaling intermediates and second messengers that trigger cell activation.34,35
The integrins comprise a family of 24 heterodimeric receptors, each of which is composed of an α subunit and another β subunit. These molecules dynamically alter their adhesive properties through conformational changes (affinity) as well as through spatial redistribution on the cell surface (avidity).36 Recent observations predict the existence of 3 conformational states (bent conformation with low affinity, extended conformation with intermediate affinity, and extended conformation with high affinity).37,38 The most relevant integrins for leukocyte adhesion to the endothelium are members of the β2 subfamily, particularly LFA-1 (CD11a/CD18 or αLβ2) and myeloid-specific integrin Mac-1 (CD11b/CD18 or αMβ2), as well as integrins α4 VLA-4 (a4β1) and α4β7. Most of the ligands are transmembrane proteins that belong to the immunoglobulin superfamily. LFA-1 can bind to 5 intercellular adhesion molecules (ICAM-1 to ICAM-5), although the most important of these are ICAM-1 and ICAM-3.39 ICAM-1 is expressed on leukocytes, dendritic cells, and epithelial cells. In addition, expression is low on quiescent endothelial cells and increases with proinflammatory stimuli.40 ICAM-3 is expressed constitutively on all leukocytes.41 In addition to LFA-1, another ligand is the junctional adhesion molecule JAM-A, which is selectively concentrated on the apical region of the tight junctions of the endothelium and is partially redistributed to the apical surface of the endothelium with certain proinflammatory stimuli.42 On the other hand, Mac-1 interacts with ICAM-1, JAM-C, and the receptor for advanced glycation endproducts (RAGE).43,44 The integrin VLA-4 interacts with VCAM-1,45 which is an adhesion molecule that is expressed de novo after endothelial activation46 and also binds to JAM-B.47 VLA-4 also interacts with ADAM-28, fibronectin, osteopontin, thrombospondin, the von Willebrand coagulation factor, and the invasin bacterial protein.48
Finally, αβ7 integrin, apart from interacting with VCAM-1 and fibronectin, specifically recognizes MAdCAM-1, a receptor expressed in the lymphoid tissues of the mucosa.19
Modulation of Chemokine-Mediated Integrin Activity
As tethering to the vascular endothelium occurs, the rolling velocity of the leukocytes slows and they are activated on encountering immobilized chemokines and integrin ligands exposed on the apical endothelial surface. This activation step enables the arrest of leukocytes and their subsequent firm adhesion to the endothelium under physiological flow conditions.49,50 Leukocyte activation implies a marked morphological change: the rounded circulating cell is transformed into a promigratory cell with polarized morphology, in which at least 2 regions can be identified, the cell front and the cellular uropod.51 The polarization of the leukocytes allows the cell to coordinate the intracellular forces to produce the necessary cell crawling during the extravasation process.52
The chemokines bound to the glycosaminoglycans of the apical endothelial membrane act by signaling via the G protein coupled receptors (GPCR) located on the microvilli of the leukocyte, thereby inducing a wide range of "outside in" signals in a fraction of a second. These lead to multiple conformational changes in the integrins.53-55 The complexity and the short time period of the signaling mechanisms induced by the chemokines that control the activation of the integrins are consistent with the existence of compartmentalized and pre-formed protein networks ("signalosomes") in the leukocytes.56 The presence of specific chemokines in different vascular beds helps orchestrate the selective recruitment of different leukocyte subpopulations to the inflammatory foci or to the secondary lymphoid organs.57 In addition, chemokines can exert a differential effect on specific integrins within the same microenvironment.58
Modulation of Ligand-Mediated Integrin Affinity
After chemokine-induced activation, the conformation of the integrins changes reversibly from the inactive (bent) form to the extended form with intermediate affinity. This event prepares the integrin for binding to its endothelial ligand. The integrins that contain an I domain inserted into their α subunits undergo a subsequent conformational change after binding to the ligand, culminating in the complete activation of the integrin and leukocyte arrest.59-61 Therefore, the high-affinity conformational state for immediate arrest of the leukocyte on the endothelium requires immobilized chemokines and the integrin ligands.55,62 However, the α4 integrins, which contain an I-like domain on the β chains, can spontaneously interact with their endothelial ligands without a prior chemotactic trigger.17
The signaling induced by the binding to the ligand leads to the separation of the cytoplasmic regions of the subunits of the integrin, thereby favoring its association with the cortical actin cytoskeleton. The α4 integrins are basically linked through paxillin while β2 integrins are linked through talin, filamin, and other structural molecules. In addition, the binding to the ligand increases the recruitment of additional integrins to increase the firm adhesion of the leukocyte in conditions of flow stress.63 This clustering of integrins depends on the release from their tether to the cytoskeleton—a process mediated by protein kinase C (PKC) and calpain—to increase their lateral mobility on the membrane.64 In addition, the role of Rap-1 and its activator CalDAG-GEFI have recently been described, as well as the coordinated action of kindlin-3 with talin in the activation of integrins to mediate the leukocyte firm adhesion in different types of hematopoietic cells.65,66 With regard to the spatial organization of the integrins, it has been shown that nanoclusters of LFA-1 not bound to its ligand are present and these enable the efficient formation of microclusters induced by binding to a ligand.67,68
On the other hand, several studies indicate that flow stress also regulates the integrins, reinforcing their bonds and even increasing their affinity.69,70 The integration of signaling derived from chemokines and the external forces to favor transmigration has been defined as the phenomenon of chemorheotaxis.71
Regulation of Leukocyte Crawling by Integrins
The signals implicated in the integrin-mediated firm adhesion of leukocytes to the endothelium have to be attenuated and the original contact has to be weakened enough to allow the migration of the leukocyte towards the appropriate site to start the process of endothelial transmigration. The β2 integrins seem to play an important role in crawling, as blockade of the integrin or its ligands leads to random migration, failure to position the cell at the interendothelial junctions, and defective diapedesis.72 In vivo studies using genetically modified mice lacking LFA-1 or Mac-1 clearly delineate the different underlying mechanisms for each of these β2 integrins. Whereas firm adhesion is mediated by LFA-1, crawling depends on Mac-1; both processes contribute to an efficient migration.73 After activation by binding to a ligand, the integrins regulate different effectors of myosin contractility, actin-remodeling GTPases, and molecules implicated in the regulation of the microtubule network both at the cell front and in the uropod. Thus, the integration of signals generated at both cell poles leads to coordinated movement of the leukocyte.34
Functional Role of the Endothelial Adhesion Molecules VCAM-1 and ICAM-1 in Leukocyte Capture
VCAM-1 and ICAM-1 molecules, both members of the immunoglobulin superfamily, are the main endothelial adhesion molecules implicated in the binding to integrins VLA-4 and LFA-1, respectively.45,74 ICAM-1 is scarcely expressed on the quiescent endothelium, whereas the expression of both molecules is induced after cell activation by proinflammatory cytokines such as interleukin (IL) 1 and tumor necrosis factor (TNF) α.40,46 In addition, binding of VCAM-1 and ICAM-1 to the actin cytoskeleton has been reported through 2 members of the ERM family, namely, ezrin and meosin.75,76 These molecules act as links between the membrane and the actin cytoskeleton, regulating cortical morphogenesis and cell adhesion.
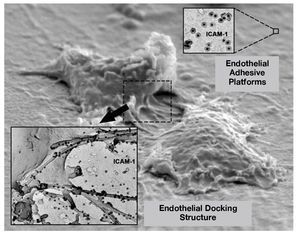
The dynamics of VCAM-1 and ICAM-1 has been studied in human umbilical vein endothelial cells (HUVECs) activated with TNF during the process of leukocyte-endothelium interaction. It has been observed that, after leukocyte arrest on the endothelium, the binding of VCAM-1 and ICAM-1 with their ligands triggers the reorganization of the endothelial cortical actin cytoskeleton and generates a 3-dimensional docking structure that surrounds the leukocyte and prevents leukocytes adhered in conditions of physiological flow from becoming unbound. This structure has a large accumulation of adhesion receptors, as well as the activated ezrin and moesin proteins. The endothelial docking structure is supported by the actin cytoskeleton, docking actin-bundling proteins such as α actinin, proteins typical of focal adhesions such as talin, paxillin, and vinculin, and actin nucleating proteins. In addition, second messengers such as PI(4,5)P2 and the Rho/160ROCK signaling pathway are also important for generating and maintaining the endothelial docking structure (Figure 2).75 Furthermore, both ICAM-1 and VCAM-1 cluster together in the endothelial docking structure, although one of them is not bound to its corresponding ligand. This joint clustering also proceeds independently of the anchoring to the actin cytoskeleton and of the formation of ICAM-1/VCAM-1 heterodimers, as this is due to the inclusion of VCAM-1 and ICAM-1 in microdomains rich in tetraspanins, which act as endothelial platforms for specialized adhesion77 (Figure 2). The tetraspanins are small proteins that cross the membrane 4 times with lateral interaction of their second extracellular domain with other integral proteins of the membrane, regulate membrane function, and form multiple protein domains on the plasma membrane. They have been implicated in several cell functions, including migration, homotypic and heterotypic cell-cell adhesion, and antigen presentation, viral infection, and gamete fusion.78-82
Figure 2. Active role of the endothelium during extravasation. The scanning electron microscope image shows the organization of endothelial adhesion receptors in nanoclusters on the apical membrane (endothelial adhesive platforms; staining corresponds to ICAM-1 using antibodies coupled to colloidal gold). When a leukocyte establishes contact with the endothelium, the endothelial adhesion receptors are concentrated in the endothelial docking structure, which keeps the leukocyte firmly adhered and prevents it from becoming separated due to the force of the flow that it has to withstand.
The use of innovative microscopy analytical techniques has enabled the characterization of the diffusive properties, organization at the nano scale, and specific molecular interactions within the microdomains in live primary human endothelial cells. Such studies have provided convincing evidence of the existence of endothelial adhesion platforms as physical entities distinct from the lipid rafts in the plasma membrane.77 Scanning electron microscopy in samples treated with a specific peptide blocker of tetraspanins reveals the nanoclustering or avidity of VCAM-1 and ICAM-1 induced by the endothelial adhesion platforms as a new supramolecular organization mechanism that regulates the efficient adhesive capacity of both endothelial adhesion receptors to their counter-receptors, the leukocyte integrins.77 The functional relevance of the inclusion of ICAM-1 and VCAM-1 in tetraspanin microdomains in endothelial cells has been demonstrated through the use of an experimental strategy with interfering RNA targeting the tetraspanins CD9 and CD151 in primary human endothelial cells and through competitive blockade with glutathione-S-transferase (GST) fusion proteins that contain the second extracellular region of CD9.83 Therefore, the inclusion of ICAM-1 and VCAM-1 in tetraspanin domains is necessary for these domains to function properly in stringent dynamic conditions such as flow stress. VCAM-1 and ICAM-1 are not the only adhesion receptors that interact with tetraspanin microdomains, others such as JAM-A, PECAM-1, ICAM-2, or CD44 also do so. It could therefore be postulated that the tetraspanin microdomains act as specialized platforms that constitutively organize the appropriate adhesion receptors in the membrane for fast kinetics and efficient leukocyte extravasation.77
The endothelial adhesion receptors VCAM-1 and ICAM-1 are able to transmit signals after binding to the ligand. The VCAM-1 molecule is implicated in opening the interendothelial junctions to facilitate leukocyte extravasation. In fact, VCAM-1 induces the activation of NADPH oxidase (possibly NOX2) and production of reactive oxygen species (ROS) dependent on GTPase rac activity, with the subsequent activation of matrix metalloproteinases and loss of adhesion mediated by VE-cadherin due to phosphorylation of β-catenin by Pyk-2,84-89 thereby favoring the extravasation process. On the other hand, VCAM-1 and ICAM-1 are able to induce a rapid increase in Ca2+ concentration, leading to the activation of Src kinase and the subsequent phosphorylation of cortactin.90-93 ICAM-1 can also activate RhoA, inducing the formation of stress fibers and the phosphorylation of focal adhesion kinase (FAK), paxillin, and p130Cas, which in turn are implicated in signaling routes involving c-Jun N-terminal kinase (JNK) and p38.94-97 This increases endothelial permeability and is associated with increased transendothelial leukocyte migration. The induction of c-fos and rhoA transcription has also been reported via ICAM-1.96 Finally, ICAM-1 can induce its own expression and that of VCAM-1, acting as a regulating mechanism to facilitate leukocyte transmigration.98
INTEGRINS AND THEIR LIGANDS DURING ENDOTHELIAL TRANSMIGRATION
During endothelial transmigration, the endothelial junctions are partially dismantled to avoid damage to the monolayer or substantial changes in permeability. Thus, the leukocyte membranes and the endothelium remain in close contact during diapedesis and, afterwards, the endothelial membranes reseal their links.
Once the leukocytes have reached an appropriate site for transmigration (preferably the intercellular junctions), they deploy exploratory pseudopods between 2 adjacent endothelial cells. The pseudopods then transform into a lamella that moves across the open space on the monolayer. During this process, the LFA-1 molecule is the integrin with the predominant role. This molecule is quickly relocalized to form a ring-shaped cluster at the contact interface between the leukocyte and endothelium, where it interacts with ICAM-199 and, in some other cell models, with JAM-A.100 When the transmigration process is over, LFA-1 is finally concentrated in the uropod.101 Other proteins implicated in the transmigration process are ICAM-2, JAM-B, JAM-C, PECAM-1 (CD31), ESAM, and CD99. Many of these are able to interact both homophilically and heterophilically maintaining the interendothelial junctions or the leukocyteendothelial interactions.102-105
In the leukocyte transmigration process, in addition to the classic diapedesis route, in which the leukocytes cross the interendothelial junctions (paracellular route), there is increasing evidence of an alternative route in which the leukocytes can migrate through individual endothelial cells (transcellular route) without perturbing the interendothelial junctions. This process takes place preferentially in the microvasculature, the blood-brain barrier, or high endothelial venules of the secondary lymphoid organs rather than in the macrovasculature.106-108 Recent observations on the mechanism of this transcellular migration process indicate that, initially, the leukocytes generate invasive podosomes dependent on Src kinase and Wiskott-Aldrich syndrome protein (WASP) activity to palpate the endothelial surface. These podosomes subsequently develop into the transcellular pore. In the endothelium. It is necessary membrane fusion regulated by calcium and SNARE-containing complexes, as well as new membrane supply by vacuole-vesicular organulles.108 It has been also reported the translocation of ICAM-1 to caveolae after leukocyte adhesion and the subsequent formation of a kind of multivesicular channel containing ICAM-1 and caveolin-1 around a leukocyte pseudopod that penetrates through the endothelial cell. Both proteins, ICAM-1 and caveolin, follow the path of the entire leukocyte, moving towards the basal endothelial membrane.109 In addition, the intermediate filament protein vimentin also seems to play an important role in the transcellular route.110 The in vivo presence of dome-shaped endothelial structures that cover the leukocyte during transendothelial migration have also recently been described.111 These observations seem to indicate that the endothelial docking structures might become domes that completely envelope the leukocytes on the luminal face of the endothelium, thereby allowing rupture of the basolateral membrane without compromising the endothelial barrier function.
ANTIADHESION-BASED THERAPIES
The advances in our knowledge of the molecular mechanisms that underlie cell migration and the extravasation cascade have allowed molecular targets to be identified for antiadhesion therapy for inflammation. Monoclonal antibodies against the α4 and αL chains have shown a clear beneficial effect in different animal models of inflammatory and autoimmune states, as well as in human diseases such as multiple sclerosis, inflammatory bowel disease, and psoriasis. Similar results have been obtained in animal models with different VLA-4 synthetic peptides.
The promising results obtained in these animal studies have encouraged formation of to different groups and pharmaceutical companies dedicated to developing new drugs for clinical trials. Thus, a humanized anti-VLA-4 monoclonal antibody has shown a clear therapeutic effect in relapses of multiple sclerosis112 and Crohn disease.113 Other potential uses of this type of therapeutic monoclonal antibody would be in inflammatory and/or autoimmune diseases that are widespread in the general population, such as rheumatoid arthritis, asthma, and type 1 diabetes mellitus.114,115 As in the case of VLA-4, the anti-LFA-1 antibody has been shown to have a significant therapeutic effect in humans, as a humanized monoclonal anti-aL antibody has been approved to treated moderate to severe psoriasis.116
There is no doubt that the therapeutic monoclonal antibodies directed against adhesion molecules or costimulators represent an important step forward in treating inflammation and autoimmune diseases. However, biological agents, such as monoclonal antibodies against a4 and αL leukocyte integrin chains are directed against receptors with a range of different biological functions: the generation of immune response, differentiation of lymphocytes into Th1/Th2,117 the effector phase of immune cells, and the extravasation of leukocytes to the inflammatory foci, among others. In addition, it is evident that some monoclonal antibodies may act as agonist molecules, thereby generating intracellular signals after binding to their antigen. Thus, long-term administration of these types of drugs could have unexpected and even undesirable consequences. It would be very important to take into account all information derived from both basic studies and preclinical research to enable the appropriate design of future clinical trials with this type of biologic agent (reviewed González Amaro et al118).
A large number of anti-inflammatory therapies against different target molecules in the endothelium have also been investigated. The efficacy of blockade of P-selectin to prevent damage caused during ischemia-reperfusion processes (transplantation, thrombosis, stroke, etc) as well as the beneficial effects of anti-ICAM-1 antibodies for preventing restenotic lesions in animals have been investigated and, as a result, they are emerging as possible therapeutic targets in humans.119,120 There are also numerous studies of chronic autoimmune or inflammatory diseases in which therapies based on anti-TNF, anti-VCAM-1, or anti-ICAM-1 have been applied. Recent research points to tetraspanins and, specifically, CD9, as a potential general anti-inflammatory target that could regulate the adhesive function of multiple adhesion receptors and be more effective than individual inhibition of each one separately. However, the tetraspanin CD9 is ubiquitously expressed throughout the organism, and so the release of CD9 blockers should be done locally and restricted to the area of inflammation. Prior studies on knock-out mice lacking expression of CD9 and other tetraspanins are necessary to clarify whether this hypothesis is plausible. Indeed, recently, the role of the endothelial tetraspanin CD81 was described as a possible diagnostic and therapeutic marker of atherogenesis in humans. The expression of CD81 on the luminal surface of the endothelium increases in the initial states of the disease, and so this molecule could play a crucial role in the formation of atherosclerotic plaque by favoring adhesion of monocytes in the stage prior to the triggering of the inflammatory response.121
Section sponsored by Laboratory Dr Esteve
Financed by project RD06/0014-0030 of the Thematic Networks for Collaborative Research into Cardiovascular Diseases (RECAVA). The Centro Nacional de Investigaciones Cardiovasculares is funded by the Ministry for Health and Consumer Affairs and the Fundación Pro-CNIC.
Correspondence:
Prof. F. Sánchez-Madrid.
Servicio de Inmunología. Hospital Universitario de la Princesa. Universidad Autónoma de Madrid. Diego de León, 62. 28006 Madrid. España.
E-mail: fsanchez.hlpr@salud.madrid.org