The diagnosis and management of mitral and tricuspid valve disease have undergone major changes in the last few years. The expansion of transcatheter interventions and widespread use of new imaging techniques have altered the recommendations for the diagnosis and treatment of these diseases. Because of the exponential growth in the number of publications and clinical trials in this field, there is a strong need for continuous updating of local protocols. The recently published 2021 European Society of Cardiology guidelines for the management of valvular heart disease did not include some of the new data on these new therapies and, moreover, the number of mitral and tricuspid interventions varies widely across Europe. Therefore, all this information must be summarized to facilitate its use in each specific country. Consequently, we present the consensus document of the Section on Valvular Disease, Cardiovascular Imaging, Clinical Cardiology, and Interventional Cardiology Associations of the Spanish Society of Cardiology for the diagnosis and management of mitral and tricuspid valve disease.
Keywords
This article represents the consensus document of the Section on Valvular Disease and the Cardiovascular Imaging, Clinical Cardiology, and Interventional Cardiology associations of the Spanish Society of Cardiology for the diagnosis and treatment of mitral and tricuspid valve disease.
MITRAL VALVE DISEASEMitral regurgitation (MR) is the second most common valve disease in Europe1 and the most common one worldwide.1,2 It is classified as primary or secondary. Primary MR is caused by injury to 1 or more components of the valve apparatus, while secondary (or functional) MR is caused by changes to the ventricular or atrial geometry that result in increased tension and abnormal closure of structurally normal leaflets.3,4
Patients with primary MR may be asymptomatic for years because left ventricle (LV) volume overload results in compensatory mechanisms, such as left ventricular dilatation. If not dealt with in time, however, chronic overload will lead to increased wall stress, remodeling, myocardial fibrosis, and, eventually, ventricular dysfunction.3
Prognosis in severe MR depends on several variables, in particular, the presence of symptoms, an LV ejection fraction (LVEF) <60%, and an LV end-systolic diameter >40mm.5 Multiple studies have shown that intervention is necessary to prevent worse outcomes in asymptomatic patients with preserved LV systolic function.5,6 Some authors consider that current thresholds already correspond to a decompensated stage of LV dysfunction and call for even earlier treatment of MR.7,8
Secondary MR can be caused by LV dysfunction, myocardial infarction affecting the posterior papillary muscle, or annular dilatation secondary to severe atrial dilation, usually in the context of atrial fibrillation or heart failure (HF) with preserved LVEF.4
Treatment should always seek to reverse LV remodeling and include medical treatment and cardiac resynchronization and coronary revascularization where indicated.9,10
Because, however, regurgitation of any degree has a negative impact on prognosis (increased incidence of HF and death),11,12 valve repair or replacement may be useful in certain cases.9,10
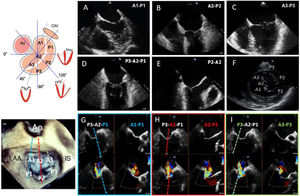
DIAGNOSISThe mitral valve apparatus is composed of an annulus, leaflets, a subvalvular apparatus (chordae tendineae and papillary muscles), and the LV. The mitral annulus is a 3-dimensional saddle-shaped structure with an oval morphology (anteroposterior and intercommissural diameters). The anterior and posterior leaflets have a similar surface and join at the anterolateral and posteromedial commissures, between which runs the coaptation line. The posterior leaflet is divided by 2 small indentations and, according to the Carpentier classification, it divides the leaflets into 3 opposing posterior-anterior scallops from the lateral to the medial sides: A1-P1, A2-P2, and A3-P3 (figure 1).
Mitral valve assessment by TEE. With 2-dimensional TEE, visualization in different planes (A-F; midesophageal 0° with varying depths, bicommissural, outflow tract, and transgastric short axis) is necessary for systematic assessment. Three-dimensional TEE permits a full “surgeon's view” assessment. Two-dimensional orthogonal views of pairs of scallops (X-plane. G, A1-P1, blue; H, A2-P2, red; I, A3-P3, green) is very useful for monitoring percutaneous procedures. Ao, aorta; IS, interatrial septum; LAA, left atrial appendage; TEE, transesophageal echocardiography.
Transthoracic echocardiography is the imaging modality of choice for patients with MR. Transesophageal echocardiography (TEE) is reserved for cases with an inconclusive diagnosis in which greater detail is required, and it is also used for planning valve repair procedures. Three-dimensional TEE provides additional information in patients with complex injuries and is also used to guide transcatheter interventions. Evaluation of MR severity is complicated. MR depends on hemodynamic conditions and no single parameter provides conclusive results. Assessment should be based on the stepwise integration of qualitative, semiquantitative, and quantitative parameters12 (table 1).
Evaluation of mitral regurgitation
| Mild (1 or 1+) | Mild to moderate (2 or 2+) | Moderate to severe (3 or 3+) | Severe (4 or 4+) | |
|---|---|---|---|---|
| Quantitative parameters | ||||
| Valve morphology | Normal/slightly abnormal leaflets or slight tenting | Moderately abnormal leaflets or moderate tenting | Moderately abnormal leaflets or moderate tenting | Flail/wide coaptation defect or severe tenting |
| Regurgitant jet on color Dopplera | Small, central (<4cm2 or <20% LA) | Moderate (4-6cm2 or 20%-30% LA) | Moderate (6-8cm2 or 30%-40% LA) | Wide central jet (>8cm2 or >50% LA) or eccentric jet with Coandă effect |
| Flow convergence,b continuous Doppler signal | None, small, or parabolic | Dense, partial, or parabolic | Dense, partial, or parabolic | Holosystolic, dense, triangular |
| Semiquantitative parameters | ||||
| Vena contracta, mmc | <3 | 3-5 | 5 to <7 | ≥7 (≥8 biplane)d |
| Pulmonary vein flowe | Systolic dominancef | Normal, systolic blunting | Systolic blunting | Minimum, absent, or reverse systolic flowg |
| Mitral valve inflow | A–wave-dominantf | Variable | E wave >1.2m/se | E wave >1.2m/sh |
| Mitral VTI/aortic VTIe | <1 | Moderate | >1.2h | >1.2h |
| Quantitative parametersi,j,k | ||||
| EROA, mm2c | <20 | 20-29 | 30-39 | ≥40 |
| Regurgitant volume, mLl | <30 | 30-44 | 45-59 | ≥60 |
| Regurgitant fraction, % | <30 | 30-39 | 40-49 | ≥50 |
| Cardiac MRI parametersm | ||||
| Regurgitant fraction, % | <30 | 30-39 | 40-49 | ≥50 |
| Structural parameters | ||||
| LV and LA sizen | No | Normal-dilated | Dilated | Dilated |
| Pulmonary arterial pressuren | Normal | Normal-high | Normal-high | High |
2D, 2-dimensional; 3D, 3-dimensional; EROA, effective regurgitant orifice area; LA, left atrium; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; PISA; proximal isovelocity surface area; TEE, transesophageal echocardiography; MR, mitral regurgitation; MRI, magnetic resonance imaging; TI, velocity-time integral.
Assessment of color flow area is used in the detection of MR. Grading based on this parameter only, however, is not recommended.
Vena contracta width and PISA-EROA should be assessed where possible. Vena contracta width can be measured in patients with eccentric or multiple jets, although the values are not additive. PISA can be used for central or eccentric jets. Measurement of vena contracta width on 3D color Doppler can help define the morphology of the regurgitant orifice.
The presence of reverse systolic flow in the pulmonary veins and an altered mitral to aortic VTI ratio are more indicative of severe MR.
MR is classified as mild, moderate, or severe. Moderate MR is further classified as mild to severe and moderate to severe. In secondary MR, an EROA ≥30mm2 or a regurgitant volume ≥45mL may indicate severe MR and has been associated with clinical events and the need for intervention.
Discrepancies may be observed between EROA values, regurgitant volume, and regurgitant fraction in situations of high or low flow.
Although there is only moderate correlation between different imaging modalities, cardiac MRI can be used to grade MR severity; the indirect method (comparison between LV stroke volume and anterograde aorta flow) is the most reproducible method. Cardiac MRI and 3D echocardiography enable a more accurate and reproducible assessment of the left cavities, although TEE measurements continue to be used for determining need for intervention.
Unless for other reasons, LV and LA size and pulmonary pressure are normal in patients with mild MR. Patients with acute, severe MR tend to have high pulmonary pressure and a normal LV size. The LV is typically dilated in severe chronic MR. Accepted cutoff values for nonsignificant LV dilatation: LVEDD <56mm, indexed LVEDV <82mL/m2, LVESD <40mm, indexed LVESV <30mL/m2, LA diameter <39mm, and LA volume <36mL/m2.
Cardiac computed tomography (CT) is essential for planning transcatheter mitral valve replacements, regardless of technique (prosthesis, valve-in-valve, valve-in-ring, valve-in-MAC [mitral annulus calcification], or native valve replacement). Cardiac CT scans are useful for prosthesis size selection, detailed visualization of the deployment area, and neo-LV outflow tract (LVOT) assessment via virtual valve implantation.13 A neo-LVOT area <1.7cm2 is predictive of LVOT obstruction, a contraindication for transcatheter mitral valve replacement.14
TREATMENTTreatment of MR depends on the underlying mechanism.5,15
Primary MRMedical treatment for acute primary MRNitrates and diuretics should be used to reduce filling pressures, and sodium nitroprusside to reduce afterload and regurgitant fraction. Inotropic agents and ventricular assist devices should be used in hemodynamically unstable patients.
Surgery or transcatheter therapy for severe acute primary MRSurgery and transcatheter therapy should be contemplated in patients with severe acute primary MR once supportive measures have been implemented to achieve stability. Emergency surgery (mostly valve replacement) is the classic treatment of choice, but it is associated with high morbidity and mortality.16 Favorable results have been reported for transcatheter valve repair in recent years, with positive outcomes in both stable patients and patients in cardiogenic shock.17,18
Medical treatment for chronic primary MRThere is no evidence to support the prophylactic use of vasodilators in patients with MR and preserved LV systolic function. The current European Guidelines for the Diagnosis and Treatment of Heart Failure recommend treating MR in patients with HF.15
Surgery or transcatheter therapy for severe chronic primary MRCurrent guidelines on the treatment of severe chronic primary MR recommend surgery (valve repair) for symptomatic patients at low surgical risk and asymptomatic patients with reduced LV function (end-diastolic diameter [EDD] ≥40mm or LVEF ≤60%) or preserved LV function if they have atrial fibrillation, a left atrial end-systolic volume ≥60mL/m2 or diameter ≥55mm, or systolic pulmonary hypertension (pulmonary artery systolic pressure >50mmHg). Transcatheter edge-to-edge repair (discussed in the next section) must be considered in patients at high surgical risk.
Secondary MRMedical treatmentThe mainstay treatment for secondary MR in patients with HF and reduced LVEF is drug therapy with β-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers, and, where appropriate, sacubitril-valsartan and sodium-glucose cotransporter 1 (SLGT1) inhibitors, cardiac resynchronization therapy (for patients with a widened QRS), and coronary revascularization (for patients with ischemic HF).
Surgery and transcatheter therapy for secondary MROptions for patients with persistent symptoms after optimal medical treatment:
- •
Surgical valve replacement in patients with heart disease amenable to surgery or patients who need another type of heart surgery.
- •
Edge-to-edge mitral valve repair in patients with suitable criteria. In the absence of suitable criteria consider other transcatheter valve procedures and suitability for ventricular assist device placement or a heart transplant (see next section).
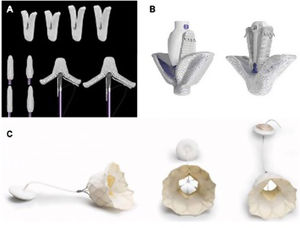
Transcatheter mitral valve repair therapies have increased exponentially in recent years, and their excellent results have forced a re-examination of the indications for MR intervention. Transcatheter replacement therapies are designed to emulate surgical repair techniques, such as annuloplasty, increase of mitral leaflet coaptation, and neochordal insertion. Transcatheter valve replacement is also now possible. A number of notable devices are already in widespread clinical use, such as the edge-to-edge repair devices MitraClip (Abbott Vascular, USA) and PASCAL (Edwards Lifesciences, USA) and the replacement system Tendyne (Abbott Vascular)19,20 (figure 2).
Selection of candidatesThe main candidates for transcatheter treatment of MR according to the position statement of the Spanish, Italian and Portuguese interventional societies21 are shown in table 2.
Candidates for transcatheter treatment of MR
| Suitable candidates |
| Severe symptomatic secondary MR + LVEF <50% + not programmed for grafting, essentially if COAPT criteria are meta |
| Severe symptomatic MR (primary or secondary) + previous patent left mammary artery graft |
| Symptomatic primary MR + high surgical risk or inoperable or not suitable candidate for surgery |
| Consider in |
| Secondary atrial MR in an unsuitable surgical candidate |
| Unsuccessful annuloplasties |
| Severe symptomatic MR after myocardial infarction in an unsuitable candidate for surgery |
| Bridging strategy for patients with severe secondary MR and a high functional class awaiting heart transplant or ventricular assist device implantation (MitraBridge) |
| Anterior mitral systolic movement in patients with hypertrophic cardiomyopathy who are not candidates for myomectomy |
LVEF, left ventricular ejection fraction; MR, mitral regurgitation.
The first trial to compare conventional surgery and transcatheter repair in MR, EVEREST II, ??showed the first-generation MitraClip device to be less effective but safer than surgery in a population with mainly primary MR.22 New-generation devices are much improved, with more than 90% of patients with primary MR treated with a fourth-generation device achieving grade 0-1 regurgitation by day 30.23
The results of the MITRA-FR24 and COAPT25 trials in which patients with secondary MR were randomized to medical treatment vs transcatheter edge-to-edge valve repair with MitraClip were published in 2018. Although no improvements were observed in the French intervention group (MITRA-FR), in the COAPT trial, patients randomized to the MitraClip group had significantly fewer hospitalizations and a 38% reduction in mortality at 2 years. The disparate results may be due to a series of differences between the 2 trials, such as MR severity, ventricular volumes, optimal medical treatment, and clinical selection of candidates (exclusion of patients with very poor prognostic factors).
Based on the findings of these trials, transcatheter mitral valve repair should be avoided in patients with very advanced disease (greater ventricular dilatation and very poor function) who have nonsevere secondary MR and are not on optimal medical treatment. Patients should be in earlier stages, have significant MR (effective regurgitant orifice area >30mm2), and be receiving optimal medical therapy. Appropriate anatomic selection and extensive experience are key to procedural success (table 3). Secondary analyses of the COAPT trial showed improved outcomes in practically all intervention subgroups analyzed. Correction of MR was even associated with significant improvements in functional class and quality of life in patients with the most similar phenotype to that observed in MITRA-FR trial.26
COAPT inclusion criteria
| Inclusion criteria (all must be present) |
| 1. Severe symptomatic mitral regurgitation |
| 2. Optimal treatment |
| 3. Ambulatory NYHA II, III or IV |
| 4. Hospitalization due to heart failure in past 12 months, BNP≥300pg/mL or NT-proBNP ≥1500 pg/mL |
| 5. Mitral surgery not an option |
| 6. LVEF 20%-50% |
| 7. End-systolic diameter ≤70mm |
| 8. Primary central jet and high likelihood of success according to implant team |
| 9. CK-MB obtained within previous 14 d is under the upper limit of normal |
| 10. Transeptal access is feasible |
| 11. Age >18y |
| 12. Informed consent provided and agreement to complete study protocol |
| Exclusion criteria (all must be absent) |
| 1. Untreated coronary disease requiring revascularization |
| 2. CABG, PCI, or TAVI in previous 30 d |
| 3. Aortic or tricuspid valve requiring surgery or transcatheter treatment |
| 4. COPD requiring home oxygen therapy |
BNP, brain natriuretic peptide; CABG, coronary artery bypass graft; CK-MB, creatine kinase-MB; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain BNP; NYHA, New York Heart Association class; PCI, percutaneous coronary intervention; TAVI, transcatheter aortic valve implantation.
The evidence from the COAPT trial is conclusive, and both US and European guidelines recommend mitral valve repair as the first-choice treatment in patients with secondary MR requiring intervention.5,27
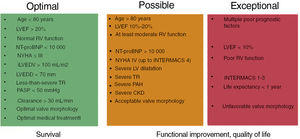
We have synthesized the above evidence into 3 levels of recommendation to guide the selection of candidates (figure 3).
Levels of recommendation for selecting patients for edge-to-edge repair. The green box shows factors associated with improvement in clinical events. Patients in this group should practically always be offered the option of edge-to-edge repair (COAPT-type patients, for example, would be expected to experience improved survival outcomes following this procedure.). The orange box shows poor prognostic factors. Patients in this group would also be expected to benefit from edge-to-edge repair, but to a lesser extent. The procedure can lead to improved quality of life and functional class, 2 powerful reasons for recommending this treatment where possible. Finally, patients with the factors shown in the red box would almost definitely not derive any benefit from mitral repair. CKD, chronic renal disease; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support scale; LV, left ventricle; LVEDD, left ventricular end-diastolic diameter; iLVEDV, indexed left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; NT-proBNP, amino-terminal fraction of pro-brain natriuretic peptide; PAH, pulmonary arterial hypertension; PASP, pulmonary artery systolic pressure; RV, right ventricle; TR, tricuspid regurgitation; NYHA, New York Heart Association functional class.
The recently developed MitraScore scale is also a useful tool for guiding patient selection, as it identifies patients with the worst prognosis and fewest chances of recovery.28
Transcatheter mitral valve repair is also used as a bridging strategy for patients awaiting advanced treatments, such as a heart transplant or ventricular assist device placement (MitraBridge strategy).29
Good outcomes have also been reported for transcatheter repair techniques in patients with favorable anatomic conditions and acute MR after myocardial infarction; one study even suggested a better impact on prognosis compared with surgery.18,30
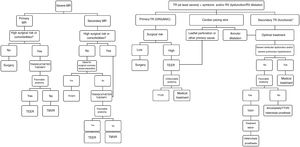
A treatment algorithm for primary and secondary MR summarizing the above information is given in Figure 4.
Central illustration. Algorithm for the treatment of MR and TR. MR, mitral regurgitation; TEER, transcatheter edge-to-edge repair; TMVR, transcatheter mitral valve repair; TTVR, transcatheter tricuspid valve repair; RV, right ventricle; TR, tricuspid regurgitation.
*The tricuspid valve should be treated in patients with severe TR secondary to left heart surgery.
The criteria for choosing between mitral valve repair and replacement are summarized in table 4. The Tendyne replacement system (Abbott Vascular) achieves favorable outcomes in properly selected patients.31,32
Factors that favor transcatheter mitral valve replacement over repair
| Unfavorable mitral valve anatomy, precluding reduction of MR to grade 0-1 with edge-to-edge repair |
| High probability of mitral stenosis with edge-to-edge repair |
| Several mitral annular calcification |
| LVEF <30% |
| Hemodynamic stability |
LVEF, left ventricle ejection fraction; MR, mitral regurgitation.
Moderate to severe tricuspid regurgitation (TR) affects up to 4% of patients older than 75 years.33 It is thus a highly common condition whose prevalence is directly proportional to age.34
Chronic TR leads to right ventricular (RV) volume overload, which results in RV remodeling and contributes to progressive worsening of the condition.34 This self-perpetuating process is associated with worse survival outcomes and worsening signs and symptoms of congestion, whether pulmonary pressure or LV systolic function.35-37
TR has traditionally been considered a benign condition, probably because symptoms linked to significant RV dysfunction or dilatation and pulmonary hypertension develop late. Surgery at late stages of disease carries a high risk, with in-hospital mortality rates ranging from 10% to 35% in patients with a history of heart surgery who undergo tricuspid valve surgery.38 These high rates have been attributed to late intervention.
The emergence of transcatheter therapies for aortic and mitral valve disease triggered increasing interest in their use in TR and an exponential growth in studies investigating transcatheter approaches for a supposedly benign disease that has classically been undertreated.
The novel treatment options are discussed in the sections below, and summarized in a diagnostic and treatment algorithm mainly designed to guide optimal timing and choice of treatment in patients with severe TR and emphasize the importance of intervening before the condition progresses to an advanced stage.
DIAGNOSISEchocardiography remains the first-line imaging modality for the diagnosis and management of TR. The tricuspid valve apparatus is composed of leaflets, the annulus, the subvalvular apparatus (chordae tendineae and papillary muscles), and the RV. Diagnosis of TR requires a description of disease severity and underlying causes and mechanisms, anatomic assessment of the right cavities, and estimation of pulmonary pressures.12,39
Cardiac magnetic resonance imaging (MRI) serves as a diagnostic alternative when sufficient anatomic definition of the tricuspid valve is not possible with TEE. CT allows for better assessment of the tricuspid annulus and degree of annular calcification, crucial information when planning a transcatheter procedure. Initial imaging with 2- and 3-dimensional transthoracic echocardiography should always be used to determine RV size and function, but cardiac MRI is the gold standard for full assessment.12,40-42 The current classification of the causes and mechanisms underlying TR is provided in table 5.12,41,43
Mechanisms and causes of tricuspid regurgitation
| Leaflet anatomy | Pathologic | Normal | Normal | Normal | Pathologic |
|---|---|---|---|---|---|
| Etiology | Carpentier ICongenitalEndocarditisCarpentier IITraumaticPostbiopsyMyxoid degenerationCarpentier IIIARadiotherapyRheumatic diseaseCarcinoid diseaseTumor | Carpentier IAnnular dilatation secondary to LA dilatation (atrial fibrillation or flutter, HF with preserved systolic function, age) | Carpentier IIIBAnnular dilatation secondary to RV dilatation (left valve disease, PHT, RV infarction, RV cardiomyopathy) | Carpentier IIIBStimulation device (pacemaker, ICD, CRT) | Carpentier IStimulation device (pacemaker, ICD, CRT) |
| Pathophysiology | Structural deficit that leading to leaflet coaptation due to excessive or restricted motion | Annular dilatation secondary to RA enlargement and dysfunction; the RV is generally normal | Annular dilatation secondary to RV enlargement and dysfunction, with tethering of leaflets | Lead displacement or interference with leaflets | Adherence, perforation, or tearing of leaflets, or rupture of subvalvular apparatus by lead |
| Diagnostic imaging | Anatomic abnormalities and altered motion of leaflets and subvalvular apparatus | Annular dilatationNormal leaflet motionRA enlargement and dysfunctionRV tends to be normal | Leaflet tetheringRestricted leaflet motion in systoleAnnular, RV, and RA dilatation and possible RV and RA dysfunction | Altered leaflet motionStructural changes to leaflets may be presentTendency for annular, RV, and RA dilatation | Adherence, perforation, or tearing of leaflets, or rupture of subvalvular apparatus by lead |
CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; RA, right atrial; RV, right ventricular.
Based on data from Hahn et al.41
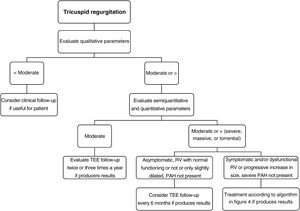
TR severity should be assessed using multiple qualitative, semiquantitative, and quantitative variables. In 2017, the TR grading system was expanded to include massive and torrential regurgitation,44 which have shown higher predictive value for HF hospitalization and mortality. The current grading system is shown in table 6. The steps for evaluating and managing a patient with TR in routine clinical practice are summarized in figure 5.12,39,41,44
Assessment of TR severity
| Grade | ||||||
|---|---|---|---|---|---|---|
| Variable | Mild | Moderate | Severe | Massive | Torrential | Role in quantification |
| Qualitative | ||||||
| Valve anatomy | Normal/abnormal | Normal/abnormal | Normal/abnormal | Normal/abnormal | Normal/abnormal | For distinguishing severe or higher-grade TR from other grades |
| Jet by color Doppler | Small central | Moderate central | Large central or eccentric | Large central or eccentric | Large central or eccentric | |
| Continuous Doppler wave assessment of regurgitant jet | Parabolic | Parabolic or triangular | Triangular with early peaking | Triangular with early peaking | Triangular with early peaking | |
| Semiquantitative | ||||||
| Vena contracta | <3 mm | 3-6.9mm | 7-13mm | 14-20mm | ≥21 mm | For distinguishing severe or higher-grade TR from other grades |
| PISA radius | ≤ 5mm | 6-9mm | >9mm | |||
| Hepatic vein flowe | Systolic dominance | Systolic blunting | Reverse systolic flow | Reverse systolic flow | Reverse systolic flow | Essential for defining severe or high-grade TR |
| Tricuspid filling | Dominant E wave ≥1 m/s | Dominant E wave ≥1 m/s | Dominant E wave ≥1 m/s | |||
| 3D vena contracta or quantitative ROA | 75-94mm2 | 95-114mm2 | ≥115mm2 | |||
| Quantitative | ||||||
| EROA (PISA) | <20mm2 | 20-39mm2 | 40-59mm2 | 60-79mm2 | ≥80mm2 | Best parameter |
| Regurgitant volume | <30mL | 30-44 mL | ≥45mL | ≥ | ≥ | |
| Regurgitant fraction | ≤15% | 16%-49% | ≥50% | ≥ | ≥ | |
| Structural parameters | ||||||
| Dilatated cavities | +/– | + | ++ (depending on etiology) | +++ (depending on etiology) | +++(depending on etiology) | |
| Inferior vena cava | <2cm | 2.1-2.5 cm | >2.5cm | >2.5cm | ||
3D, 3-dimensional; EROA, effective regurgitant orifice area; TR, tricuspid regurgitation.
Multimodality imaging is essential for adequate TR assessment, especially in the context of transcatheter therapies. Cardiac MRI is the gold standard for right cavity assessment and is essential for determining the effects of TR on these structures. RV function can influence the feasibility of different treatment options. Cardiac CT should be used to measure calcium scores and assess extracardiac structures that can be accessed percutaneously. The strengths and uses of the different imaging techniques in the assessment of TR within the setting of transcatheter therapy are summarized in table 7. Anatomic limitations that determine choice of approach are shown in table 8.12,39,41,44,45
Role of imaging techniques in assessment of TR before transcatheter therapy
| Strengths | Limitations | Use during transcatheter therapy | |
|---|---|---|---|
| TTE (2D and 3D) | Valve anatomyMechanism, etiology, and degree of TRAssessment of RV, RA, and left cavitiesAssessment of pulmonary hemodynamicsGood temporal and spatial resolutionNo radiation | WindowRequires experiencePortability | Previous assessment and guidance during edge-to-edge repairAssessment before percutaneous annuloplastyAssessment before percutaneous prosthesis implantation |
| TEE (2D and 3D) | Valve anatomyMechanism, etiology, and degree of TRAssessment of RV, RA, and left cavitiesAssessment of pulmonary hemodynamicsGuidance during proceduresGood temporal and spatial resolutionNo radiationAutomatic postprocessing of 3D images | WindowContraindications for TEESedation or anesthesiaRequires experiencePortability | Previous assessment and guidance during edge-to-edge repairPrevious assessment and guidance during percutaneous ring implantationPrevious assessment and guidance during percutaneous valve implantation |
| Cardiac CT | Annular size, morphology, and calcificationIdentification of adjacent structures and state (coronary angiography also)Identification of ideal fluoroscopy viewsAssessment of extracardiac structures (venae cava)Extracardiac vascular assessmentExcellent spatial resolution | RadiationAvailabilityNeeds iodine contrastLow temporal resolutionNonportableNot possible in patients with arrhythmias or high cardiac frequenciesArtifacts with calcium and metal devices | Assessment before annuloplasty and percutaneous tricuspid prosthesis implantationAssessment before extracardiac prosthesis implantation |
| Cardiac MRI | Assessment of TR gradeQuantification of RV morphology and function (gold standard)Tissue characterization (with or without gadolinium enhancement)Good temporal and spatial resolutionAssessment of venae cavaHemodynamic informationNo radiation | AvailabilityOptional use of gadolinium enhancementLower spatial resolution than with ultrasound or CTLower temporal resolution than with ultrasoundNot possible in patients with arrhythmias or high cardiac frequenciesCalcium not visibleIncompatible with certain devicesUnderestimates maximum speedsNot portable | Preprocedural assessment of right cavities |
| Fluoroscopy | Coronary anatomy | Radiation | Procedural guidance in combination with TEE |
| Right heart hemodynamic study | Gold standard for assessing pressures in right circuit, reversibility, and pressure response to volume overloads | Requires experienceRadiation | Essential decision-taking tool before transcatheter therapies |
2D, 2-dimensional; 3D, 3 dimensional; CT, computed tomography; RV, right ventricle; TEE, transesophageal echocardiography; TR, tricuspid regurgitation; TTE, transthoracic echocardiography.
Anatomic characteristics to guide device selection
| Technique | Ideal | Possible | Unfavorable |
|---|---|---|---|
| Edge-to-edge repair | Septolateral coaptation gap <7mmCentral jet with option of anteroseptal graspingLeaflets ≥7mm with prolapse or secondary TR with normal leaflet motionTrivalve valveNo device leads | Septolateral coaptation gap 7-8.5mmCentral jet in valve with >3 commissures and possible grasping zonesFlail or prolapse <10mmTethering height <9mm with reduced leaflet motionDevice leads but no valve damage | Gap >8.5mmSevere enlargement or length <7mm or leaflet perforationLarger flaps or tethering than possibleConsiderable changes to subvalvular apparatusNoncentral, eccentric jet or multiple jets with torrential disease (vena contracta <14mm)Device lead with valve damageSevere pulmonary hypertension |
| Annuloplasty | TR secondary to annular dilatationHeight <0.76cm, tethering area <1.63cm2 or 3D volume <2.3 mLCentral TRAcceptable anatomic landing zone | Device carrier without leaflet injuryTethering with height 0.76-1cm, area 1.63-2.5cm2 or 3D volume 2.3-3.5mL | Excessive annular dilatationTethering height >1cm, area >2.5cm2, and 3D volume >3.5 mLProximity of right coronary artery to tricuspid annulusDevice carrier with leaflet injurySevere pulmonary hypertension |
| Transcatheter tricuspid prosthetic valve | History of bioprosthesis placement or tricuspid annuloplastyDevice carrier without leaflet injuryShort or enlarged leaflets | Device carrier with injury | Excessive annular dilatationDifficult anatomic landing zoneSevere RV dysfunctionSevere pulmonary hypertension |
| Heterotopic prosthesis | Suitable vena cava sizes | <10mm between RA and origin of hepatic veinsSevere pulmonary hypertensionSevere RV dysfunction |
RA, right atrium; RV, right ventricle; TR, tricuspid regurgitation.
As mentioned, TR is an independent predictor of poor prognosis, regardless of the clinical setting.35 Novel transcatheter repair techniques offer an effective alternative for select cases and in addition are associated with a low risk of complications.
SurgeryIsolated tricuspid valve surgery for secondary TR in patients who are not candidates for mitral or aortic valve surgery is a controversial topic, and is rarely performed due to the risk profile of the patients and the high risk of postoperative complications.46,47 Transcatheter therapies are gaining importance in this setting. In one study, early tricuspid valve repair in patients with secondary TR undergoing surgery for MR was associated with a lower need for intervention during follow-up, although pacemaker implantation was more common in patients who underwent surgery and tricuspid annuloplasty than in those treated with surgery only (14.1% vs 2.5%).48
Surgery is currently the treatment of choice for the correction of primary TR. Where possible, repair is preferable to replacement. Bioprostheses are increasingly favored in patients undergoing tricuspid valve replacement, as they eliminate the need for oral anticoagulants and do not exclude the possibility of future valve-in-valve implantations in the event of bioprosthetic valve dysfunction.
Medical treatmentConservative and medical treatment options for TR are limited, and are aimed at treating the mechanisms responsible for functional regurgitation. Diuretics are the mainstay treatment for preventing RV volume overload and treating right HF manifestations. Aldosterone antagonists can be useful for treating hepatic congestion. Pulmonary vasodilators are contraindicated in patients with corrected left valvular heart disease and TR secondary to pulmonary hypertension due to their association with worse outcomes.49 Rhythm control may be useful for correcting TR caused by annular dilatation secondary to atrial fibrillation, but its effectiveness has not been clearly established.
Medical treatment should not serve as an argument to delay intervention in candidates for invasive procedures. Postponing treatment until advanced stages of disease will lower the likelihood of RV, renal, and hepatic function recovery, even if central venous and RV congestion are successfully reduced.
Transcatheter tricuspid valve therapyTranscatheter tricuspid valve repair procedures emulate surgical procedures but do not require thoracotomy or extracorporeal circulation, which have a particularly deleterious effect on patients with RV dysfunction.
Edge-to-edge repairEdge-to-edge devices are the most widely used repair systems. Their main advantage is that they can be used to treat TR caused by the presence of devices (pacemakers). Their main limiting factor is the echocardiographic window, especially in patients undergoing mitral valve surgery.
Early experience with the MitraClip device led to modifications in the device's delivery system and guide catheter to improve positioning and coaxial alignment with leaflets. The modified clip, TriClip (Abbott, USA), was evaluated in the prospective TRILUMINATE trial, which included 85 patients, 63% of whom had massive or torrential TR. By month 12, TR had been reduced to moderate or less in 71% of patients. Additional benefits were improvements in functional class (NYHA I-II, 83% vs 31% at baseline), fewer HF readmissions (0.8 vs 1.30 events/patient/y at baseline), improved quality of life (increase of 10 points on the Kansas City Cardiomyopathy Questionnaire score in 65% of patients), a decrease in right ventricular and atrial dimensions, and a reduction in major complication rates at 1 year.50 Twelve-month mortality was 7.1% overall (4.8% for cardiovascular mortality). The TriClip is currently being evaluated in the prospective bRIGHT study (NCT04483089) investigating the performance and safety of the 4 device sizes currently available (NT, XT, NTW, and XTW). The TriClip is also being compared with medical treatment in the pivotal Triluminate trial (NCT03904147).
The PASCAL transcatheter valve repair system (version P10) was evaluated in a series of 34 patients, 78% of whom had massive or torrential TR; 52% achieved a TR severity reduction to moderate or less, while 85% achieved a reduction of at least 1 grade.51 The ACE version of the PASCAL system (which has narrower clasps and no spacer) is currently being evaluated and is expected to further reduce TR.
AnnuloplastyThe Cardioband direct annuloplasty device (Edwards Lifesciences), designed to emulate a flexible surgical ring, is implanted by means of an adjustable band anchored via screws.52 It can be used to treat patients with wide coaptation defects that are difficult to access using the edge-to-edge technique. Anatomic limitations include a wide annulus and the proximity of the right coronary artery to the annulus. Another advantage of Cardioband is that it does not exclude future valve repairs or replacements.
In the TRI-REPAIR study analyzing 2-year outcomes of Cardioband implantation in 30 patients (76% of whom had massive or torrential TR), 72% of patients achieved a significant reduction to moderate or less.53
Transcatheter valve replacementEarly transatrial approaches were associated with high morbidity and mortality, triggering an inevitable transition to devices suitable for transfemoral implantation.
Twenty-five patients underwent tricuspid valve replacement with the EVOQUE valve (Edwards Lifesciences) in a compassionate-use study. Implantation was successful in 23 patients, all of whom achieved minimal or slight residual TR; 8% required a permanent pacemaker.54
The feasibility of valve replacement therapy extends candidacy for transcatheter TR treatment to patients with complex defects and wider annuli. Patients with severe TR should not be considered for tricuspid valve replacement as it offers little clinical benefit.
Heterotropic prosthesesTricValve (Products+Features, Germany)55 and TRICENTO (MEDIRA, Germany)56 are heterotopic caval valve prostheses that can mitigate the symptoms of TR without directly addressing the cause. They should therefore be primarily reserved for situations in which repair techniques have been ruled out or unsuccessful.
Treatment algorithm for TRConsidering that TR is a high-risk condition, the gold standard treatment should be expanded to include invasive correction alongside purely medical options.57 Surgery remains a viable option for the few young patients without significant comorbidities, but transcatheter repair is emerging as a better option for most cases (figure 4). An active search for TR should be performed in susceptible patients, that is, patients who have undergone mitral valve surgery, patients with atrial fibrillation, and patients carrying a cardiac stimulation device.
Optimal timing of treatment remains to be established, as the evidence is less robust than it is for mitral or aortic valve disease. This group advocates early consideration of transcatheter repair as a) it increases the likelihood of functional recovery of affected organs (symptom onset usually indicates end-stage disease) and b) has a good safety profile.
ConclusionsWe have presented the consensus document on the diagnosis and treatment of mitral and TR of the Section on Valvular Heart Disease and the Cardiovascular Imaging, Clinical Cardiology, and Interventional Cardiology associations of the Spanish Society of Cardiology.
FundingNo funding was received.
Authors’ ContributionsAll the authors contributed equally to this document.
Conflicts of InterestR. Estévez-Loureiro is a proctor for Abbott Vascular, Edwards Lifesciences, Boston Scientific, and Products and Features. D. Arzamendi is a proctor for Abbott Vascular, Edwards Lifesciences, and Boston Scientific. I. Cruz González is a proctor for Abbott Vascular, Boston Scientific, and Products and Features, and a consultant for Edwards Lifesciences.
Interventional Cardiology Association of the Spanish Society of Cardiology: I. Cruz González (president), R. Estevez Loureiro, and D. Arzamendi.
Cardiac Imaging Association of the Spanish Society of Cardiology: L. Borreguero (president), A. Martínez-Monzonis (president-elect), M. Barreiro.
Section on Valvular and Aortic Heart Disease of the Spanish Soviety of Cardiology: I. Vilacosta (presidente), C. Olmos Blanco, and J.C. Gómez Polo.
Clinical Cardiology Association of the Spanish Society of Cardiology: J.M. Gámez (president), A. Aguilera, and L.M. Rincón.