.
BackgroundInterventional cardiology has evolved dramatically over the past 30 years and invasive procedures have become the cornerstone for the diagnosis and treatment of cardiovascular diseases. In Spain, more than 64000 procedures are performed annually.1 Such interventions (ranging from routine coronary angiography to complex, percutaneous valve implantations) require the synchronized efforts of multidisciplinary teams. Since the first angioplasty in the year 1977, there has been a progressive fall in mortality among patients with ST segment myocardial infarction, associated with an increase in percutaneous interventions. Complex interventions are, however, potentially hazardous, with attendant risks and well-documented complications. Adverse outcomes may also occur as a direct result of the procedure itself; often the result of poor team working and failures in communication. This is well-recognized in the operating theatre environment, and much research has focused on addressing the impact of human factors on patient morbidity and mortality in this setting. Despite similarities between the 2 environments, adoption of practices including proactive identification of hazards, risk mitigation, and contingency planning aimed specifically to improve patient safety in the cardiac catheterization laboratory has been slow. Further, a lack of systematic attention to the causes of suboptimal performance in the interventional cardiology environment still persists. There is an urgent need to increase awareness that the catheterization laboratory is a high risk environment for both patients and health care providers. Interventional cardiologists need to learn from their surgical colleagues that simple steps can be taken to mitigate risks and could potentially impact the way individuals and teams perform in complex environments-beyond improved technology and pharmacology.
LESSONS LEARNT FROM AVIATION INDUSTRYSeveral similarities exist between aviation and interventional cardiology, but the aviation industry has a wealth of experience in the use of strategies to reduce risk.2 Similarities include:
- •
Complex procedures and processes with a series of critical steps
- •
Time-critical and safety-critical event flows and actions
- •
Unpredictability
- •
Rare deviations that may require urgent response despite happening infrequently
- •
Lengthy training
- •
Hierarchy in a team of professionals with a single person often designated as the final authority for the safe outcome of the process
- •
Highly visible outcomes when things go wrong
Despite these similarities, in medicine, there is a tendency to believe that most variations and many adverse outcomes are a result of the patient and/or their underlying disease, and not the healthcare provider or the system. However, despite the existence of evidence-based practice, national standards, professional guidelines, and local protocols, healthcare professionals consistently fail to deliver reliable and safe care to their patients because they tend to rely solely on the hard work and vigilance of clinical practitioners to minimize risk. In general, healthcare practitioners fail to acknowledge that individual performance is highly variable, that effective team work is beneficial for patients, and that there is a need to change the way we work, learning lessons from outside the healthcare system in order to minimize the risk of an error causing harm to a patient.
“Human factors” is a multidisciplinary field incorporating contributions from psychology, engineering, design, operations research, and anthropology. It covers understanding the properties of human capabilities and the application of this understanding to the design, development, and deployment of systems and services, such as those that occur in cardiac catheterization laboratories. Evidence from adverse event reporting indicates that many preventable errors in medicine are a result of failures in non-technical skills; eg, memory lapses or failure to implement an agreed plan. The causes of these errors are known; many are byproducts of useful cognitive functions and are caused by activities that rely on weak aspects of cognition. There is widespread literature to support the notion that errors can be prevented by designing tasks and processes that minimize dependency on weak cognition.3
Cognitive interventions help providers understand their own thought processes in decision making. Cognitive forcing strategies (a type of cognitive intervention) compel the provider to self-monitor his actions.4 Checklists, which are simple and cheap, are an example of a cognitive forcing strategy and are in common use in many industries to ensure that processes are carried out as designed. A checklist is “a formal list used to identify, schedule, compare, or verify a group of elements or; a checklist is used as a visual or oral aid that enables the user to overcome the limitations of short-term human memory”.4 Checklists not only help with memory recall, they ensure a minimum standard of care ie, to make sure that all elements of a process or intervention are delivered reliably. Design of the checklist is crucial; they should be specific to a particular task, short, clear, comprising of a simple check rather than an algorithm. Despite their acknowledged and demonstrated potential to improve patient outcomes (and outcomes outside of healthcare, eg, in aviation), their adoption in many fields of medical practice has been slow because of provider resistance. This is often because checklists have been forced on, rather than implemented by clinical teams to meet their own needs and those of their patients. In addition, delays in knowledge dissemination and integration, limited methodology to guide development and maintenance, together with a lack of effective technical strategies to make them available and easy to use5 have resulted in their limited adoption in medicine.
Checklists in medicine-Surgery and Intensive care leading the wayThere is a long history of the use of checklists in the assessment of patients (medical history and vital signs charts), and in general, non-medical health care professionals do use checklists and multidisciplinary teamwork in their daily practice. Doctors, however, are lagging behind in this respect. In the year 2001, Peter Pronovost, a critical care specialist at John Hopkins Hospital performed the first formal test of a checklist in medicine. At this time it was estimated that central venous catheter infections were causing 28 000 deaths per annum among patients in intensive care units in the United States. Thus, after implementing a care bundle aimed at reducing the rate of catheter-related blood stream infections in their own intensive care unit, Peter Pronovost and his team decided to test the intervention across the state of Michigan. This care bundle targeted clinicians in the use of 5 evidence-based interventions recommended by the centre for disease control. The recommended procedures were hand washing, using full-barrier precautions during the insertion of central venous catheters, cleaning the skin with 2% chlorhexidine, avoiding the femoral site if possible, and removing unnecessary catheters. The checklist required formal documentation that each of the 5 interventions was used every time a central venous catheter was inserted into a patient. One hundred and three intensive care units participated in the intervention. Within 3 months of implementation, the median rate of infection decreased from 2.7 (mean, 8.7) per 1000 catheter-days to 0 (mean, 2.3), a rate that was sustained throughout the remaining 15 months of follow-up,6 saving an estimated 175 million dollars in hospital costs and more than 1500 lives. What the checklist did was to simply force clinicians to apply evidence based practices and insert central venous catheters reliably in a standardized way, with obvious benefits to patients.
Six years later, a World Health Organization program called Safe Surgery Saves Lives was started under the leadership of Atul Gawande, a surgeon at the Brigham and Women's Hospital in Boston. Eight hospitals in 8 cities with a diverse set of socioeconomic environments were included in the program. The intervention involved a 2-step checklist implementation. Baseline data were collected and subsequently each local investigator was directed to implement a 19-item World Health Organization safe-surgery checklist.
The checklist was employed at 3 time points: before anesthesia (sign in), immediately before incision (time-out), and before the patient is taken out of the operating room (sign out). The primary end point was the composite of any major complication defined by the American College of Surgeons’ National Surgical Quality Improvement Program. Three thousand seven hundred and thirty three patients were enrolled at baseline and 3955 patients were enrolled after implementation of the program. The rate of any complication decreased from 11.0% at baseline to 7.0% (P<.001) after program implementation, and the total in-hospital mortality dropped from 1.5% at baseline to 0.8% (P=.003) after program implementation.7 The precise mechanisms of improvement are not obvious; however, checklist implementation required changes in systems and in the behavior (human factors) of individual surgical teams and this undoubtedly contributed to the impressive results. The checklist does not involve technology or equipment, it simply directs the team to focus on the procedure and the patient and carry out all the basic safety steps consistently and reliably. In the United Kingdom, the use of a safe surgery checklist is now mandatory in all hospitals for all patients undergoing an operation and compliance to the same is monitored by regulatory bodies.8
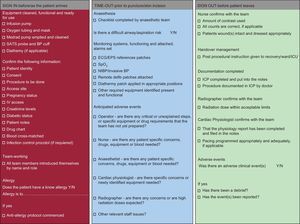
Safety in the cardiac catheterization laboratoryThe cardiac catheterization laboratory is a challenging environment with multiple professionals undertaking complex procedures, many of which may be performed under emergency conditions. In these circumstances the propensity for errors to occur is high. Examples include failure to check fasting status, renal function, or international normalized ratio prior to procedures, failure to check that vital equipment is present and working before administering sedation or anaesthesia, inadequate recording of drugs given during a procedure so that further doses are given post-operatively, and failure to compress femoral wounds for sufficient time or deploy occlusion devices effectively due to lack of experience or supervision. Many of these errors could be prevented by acknowledging the impact of human factors and implementing simple interventions (such as team briefs [Fig. 1] and checklists [Fig. 2]), thereby increasing the likelihood of a good outcome for the patient.
Catheterization laboratory safe procedures checklist. BP, blood pressure; ECG, electrocardiogram; EPS, electronic prescription service; ICP, integrated care pathway; ICU, intensive care unit; IV, intravenous; NIBP, non-invasive blood pressure; SATS, saturation; SpO2, saturation of peripheral oxygen.
The European Association of Percutaneous Cardiovascular Interventions published a recommendation to construct a checklist for the prevention of complications during percutaneous coronary intervention.9 The aim was to provide the fundamental requirements of a checklist which could be adapted by local organizations and teams for their own use. Two separate checklists were proposed, 1 for non-emergent procedures and another in case of emergent procedures. Recently, the Society for Cardiovascular Angiography and Interventions published the “clinical expert consensus statement on best practices in the cardiac catheterization laboratory”.10 The statement identifies a need for specific standards to be implemented which mirror those already employed in many surgical operating rooms across the world, including the development and use of safe procedure checklists. Despite these developments, widespread recognition of the impact of human factors in the cardiac catheterization laboratory remains poor, and implementation of such innovations is not universal.
ConclusionsThe interventional cardiology community has a great opportunity to improve the quality of care and patient safety. Despite efforts made by various scientific societies to recognize the impact of human factors and effective team behaviours, and implement standards and checklists within the cardiac catheterization laboratory, these are relatively underused. Further research is needed in this field to develop and evaluate reliable and robust methods to improve patient safety by learning from experiences in other medical specialties, and those outside medicine.
Conflicts of interestNone declared.