Keywords
INTRODUCTION
Atherosclerosis is a diffuse, systemic disease of the arterial network, the local manifestations of which are associated with clinical problems such as myocardial infarction, stroke, etc. Vulnerable lesions (high risk plaques) are characterized by a large necrotic core, a thin fibrous layer (fibroatheroma), and an inflammatory infiltrate (monocytes/macrophages, T lymphocytes, and mastocytes, etc). Although the rupture of a fibrous plaque is the main cause of intraluminal thrombosis in acute coronary syndromes and is responsible for 75% of all deaths following acute myocardial infarction, thrombosis has also been observed in eroded plaques and in those with calcified nodules.1,2 In high risk patients, plaque vulnerability is a multifocal phenomenon that involves different lesions around the coronary tree. In clinical practice, the signs of vulnerable plaques are difficult to discern. Current efforts directed towards identifying patients at risk are therefore based on three types of markers: a) images (eg, magnetic resonance, optical coherence tomography, molecular imaging, etc), for characterizing the composition of atheroma plaques and identifying those that are vulnerable; b) functional markers of vascular homeostasis (eg, arterial thickening and the endothelial-dependent vasodilatory response); and c) circulating markers (based on the measurement of markers of inflammation, oxidative stress, and thrombosis in the blood).3-6 The use of biomarkers of extracellular matrix (ECM) remodeling has been less studied, even though the ECM provides the structural and functional platform for the blood vessels; changes in its synthesis and/or breakdown must therefore play a key role in the development of atherosclerotic lesions, vascular remodeling, and plaque rupture.7,8 This has led to the proposal that certain matrix metalloproteinases (MMP), such as pregnancy-associated plasma protein A (PAPP-A),9 may serve as potential biomarkers of atherosclerotic progression. A loss of equilibrium between the molecules and factors that induce the degradation of the ECM and those that favor its synthesis and accumulation could be of prime importance in the development of clinical atherothrombotic syndromes.
PHYSIOLOGY OF THE EXTRACELLULAR MATRIX
The arterial wall is dominated by collagen type I and II, macrophages, and smooth muscle cells; these cell types govern the remodeling of the ECM. Fibronectin, laminin, elastin, and proteoglycans are also involved in ECM synthesis. The balance between ECM synthesis and breakdown is regulated by the equilibrium between the proteases that favor degradation (MMP) and their tissue inhibitors (TIMP) (Figure 1). The MMP are a family of zinc-dependent endopeptidases produced by monocytes, endothelial cells, and smooth muscle cells, whose function it is to break down numerous components of the ECM as well as other proteins not associated with it. They are synthesized and secreted as inactive proenzymes with a propeptide domain rich in cysteine able to fold and interact with the Zn++ of the catalytic domain thus impeding any enzyme activity. Activation requires the removal of the propeptide domain. Based on their structure, substrate specificity, and membrane binding (Table), these enzymes are classified as collagenases (MMP-1, 8, and 13), stromelysins (MMP-3, 10, and 11), gelatinases (MMP-2 and 9), membrane-type MMP (MT-MMP), and others (matrilysin, metalloelastase, etc).10 Their activity is regulated transcriptionally, post-translationally, and via interactions with specific inhibitors. A number of growth factors, cytokines, thrombin, and hormones increase the transcription of these enzymes, while heparin, transforming growth factor beta (TGFβ) and corticoids inhibit it.11,12 The extracellular activation of latent zymogens (pro-MMP molecules) provides a second control point. The main physiological activator of the MMP is plasmin, which converts inactive forms into active molecules by the proteolysis of the propeptide link and the exposure of the catalytic domain.13 Other enzymes, such as thrombin, factor Xa, and the MMP themselves also possess MMP-activating activity. Finally, the activity of the MMP is regulated by TIMP, of which four are known (TIMP-1, 2, 3, and 4). Inhibition is achieved via the irreversible binding of these molecules to the active enzyme. Thus, in summary, the proteolytic balance depends on the relative concentration of activators and inhibitors13 (Figure 1).
Figure 1. Synthesis and breakdown of the extracellular matrix in atherosclerosis: balance between metalloproteases (MMP) and their inhibitors (TIMP).
THE EXTRACELLULAR MATRIX AND ATHEROTHROMBOSIS
The equilibrium between MMP and TIMP is critical in the maintenance of the integrity of the cardiovascular system.13-16 This has led to the proposal that any alteration towards the breakdown of the ECM contributes to the progression of atherosclerosis and plaque instability.17 Certainly, the participation of the MMP in different mechanisms fundamental to atherothrombotic progression has been reported (Figure 2):
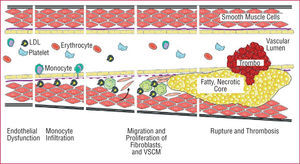
Figure. 2. The atherosclerotic process. LDL indicates low density lipoprotein; VSMC, vascular smooth muscle cells.
MMP favor monocyte infiltration of the vascular wall. An increase in the expression of certain MMP, such as MMP-12, leads to macrophage infiltration, the rupture of the internal elastic lamina, and the acceleration of the atherosclerotic process.18
The induction and activation of the MMP, particularly MMP-14 (MT1-MMP), favors the invasion of plaques by vascular smooth muscle cells and fibroblasts. The migration and proliferation of these cell types is important in the development of intimal hyperplasia.19,20
The activity of MMP-2 and 9, among others, is indispensable for the neovascularization of atherosclerotic plaques. This process, induced by proangiogenic and inflammatory stimuli, appears to be necessary for their growth, and is associated with the vulnerability of advanced lesions.21,22
The role of MMP in the formation and resolution of thrombi remains somewhat unclear (Figure 3). An abnormal reduction in the activity of ADAMTS-13 favors the appearance of thrombotic microangiopathies,23 and there is evidence of molecular interactions between MMP-3 and enzymes, substrates, and inhibitors of the fibrinolytic system. This suggests the MMP to have some role in the latter.24 They might therefore be involved in different types of thrombotic disease. Finally, thrombi themselves may be a source of proteolytic activity that might be important in the atherothrombotic process.25
Figure 3. Diagram showing the role of metalloproteases in thrombosis and fibrinolysis. ECM indicates extracellular matrix; MMP, metalloproteinase; PAI-1, plasminogen activator inhibitor type I; TAFI, thrombin activated fibrinolysis inhibitor; TIMP, tissue inhibitors of metalloproteinases; t-PA, tissue plasminogen activator; u-PA, urokinase type plasminogen activator. Modified from Lijnen.24
The physiopathological importance of changes in ECM metabolism in the development of atherothrombotic syndromes is supported by numerous pieces of evidence. It is interesting to note that intravascular thrombosis and acute myocardial infarction are rare consequences of restenosis (one of the limitations of percutaneous revascularization). Restenotic plaques appear as a consequence of the rapid proliferation of vascular smooth muscle cells and the accumulation of ECM at the lesion site. These plaques, however, rarely rupture or express MMP-9 (gelatinase B).26 In contrast, the progress of an atherosclerotic plaque from a fatty streak to an advanced, unstable element is slow, and is associated with an increase in its cellular and ECM contents as well as in proteolytic activity. This latter activity is mainly associated with the macrophages and vascular smooth muscle cells at the plaque shoulders and around the necrotic core, and is accompanied by the expression and concentration of (mostly) MMP-1 (collagenase), and MMP-9.27,28 An increase in the gelatinase MMP-9 has also been observed in the coronary plaques of patients with unstable angina compared to those with stable angina,29,30 and has been related to post-infarction ventricular remodeling.31,32 Increased concentrations of MMP-9 have also been reported in human brain tissue following ischemic and hemorrhagic ictus, and the induction of MMP-9 has been reported in the cerebral vasculature following fibrinolysis. This indicates that this enzyme may contribute to ischemic cerebral lesions and perihematomal edema as well as to cerebral hemorrhages and neurovascular lesions following fibrinolysis.33,34 These local alterations can be detected at the systemic level; patients with angiographic proof of coronary heart disease show an alteration in the balance of fibrinolysis/proteolysis in their peripheral blood.35 In addition, it has been reported that the urine MMP-9 concentration increases, while that of TIMP-1 is reduced, after an acute myocardial infarction.36
The expression and activity of MMP-9 in an atherosclerotic plaque, which is mainly associated with macrophages, may be the consequence of an increase in the production of the NADPH-dependent superoxide anion since it coincides with the expression of NADPH oxidase and the production of free radicals.37 However, the gelatinases and collagenases are not the only MMP associated with atherosclerosis; our group has recently shown an increase in MMP-10 (stromelysin 2) in advanced carotid plaques obtained by endarterectomy.38 MMP-10 has also been associated with aortic aneurysms, which are characterized by destructive remodeling of the vascular ECM and rupture of the wall.39 Finally, the TIMP-1 concentrations have been reported to increase in calcified areas of human atherosclerotic plaques,40 further indicating the inhibition of MMP activity may be related to greater plaque instability.
METALLOPROTEASES AND ATHEROSCLEROTIC RISK FACTORS
The majority of the classic risk factors for atherosclerosis have been related to changes in the concentrations of different ECM biomarkers and the overall Framingham score.41,42 The mechanisms behind this, however, are unclear. It may be that the risk factors modulate the vascular structure and stability of plaques, which in turn influence concentrations of MMP and TIMP, or via modifications in collagen production. Neither can it be ruled out that modified biomarker values are an epiphenomenon or an adaptive response to the process of atherosclerosis.
Age and Sex
Advanced age and male sex have been related to elevated concentrations of TIMP-1.41 Hormone therapy has been reported to reduce MMP-9 concentrations in post-menopausal women.43
Dyslipemia
In the clinical setting, the Framingham Heart Study reported MMP-9 to be detectable only in 20% of subjects, in whom it was associated with high levels of low-density lipoprotein cholesterol (LDL-C).42 In contrast, TIMP-1 was detected in all subjects and increased with the total cholesterol/high-density lipoprotein cholesterol (HDL-C) ratio.41 In subjects with familial hypercholesterolemia, an increase has been reported in concentrations of MMP-3 and 9, and TIMP-1, particularly in those at greater cardiovascular riskin whom the serum concentrations of MMP-3 and TIMP-1 have been associated with carotid atherosclerotic lesions.44 In addition, the abnormally high levels of MMP-9 in patients with familial hypercholesterolemia and coronary heart disease can be reduced through treatment with statins, although no correlation between the reduction of the MMP concentration and lower cholesterol concentrations is reported.45
Under experimental conditions it has been shown that, following angioplasty, the vascular expression of MMP-1 increases in a porcine hypercholesterolemic model, perhaps as a consequence of oxidative stress.46 In in vitro experiments, oxidized LDL has been shown to increase the production of MMP-1, 3, and 9, while that of TIMP-1 is reduced. In contrast, HDL reduces the production of several MMP types.47,48 In monocytes/macrophages it has been reported that oxidized LDL acts synergistically with proinflammatory factors to induce the expression of MMP-1 and MMP-9, while HDL prevents the induction of MMP-1 via these same agents.49
Diabetes, Obesity, and Metabolic Syndrome
MMP-9 and TIMP-1 are increased in patients with diabetes and metabolic syndrome.50,51 In experimental studies it has been observed that increased glucose levels induce the expression of MMP-1 and 2 by endothelial cells, and of MMP-9 by macrophages, but have no effect on TIMP-1.52 In a clinical study involving patients with diabetes, the control of blood sugar and atherosclerotic risk factors reduced TIMP-1 without modifying MMP-9 or TIMP-2.53
The concentration of TIMP-1, but not of MMP-9, has been related to body mass index.41 Finally, in a study involving obese women, a reduction in MMP-1 was noticed one year after performing stomach reduction surgery.54
High Blood Pressure
An increase in circulating MMP-9 but also of TIMP-1 has been reported in patients with high blood pressure and general thickening of the artery walls.55 High blood pressure is associated with an increase in collagen synthesis and a reduction in its breakdown.56
Tobacco and Alcohol
Smokers show increased concentrations of MMP-9 and TIMP-1, the extent of which is partly related to the duration of tobacco exposure.57 No association has been reported between the abusive intake of alcohol and circulating TIMP-1 and MMP-2 concentrations,58 although an increase in MMP-9 and 2 expression has been reported, especially in the alveolar macrophages. The latter is probably related to lung remodeling in the context of acute respiratory distress syndrome.59 An inverse association has been reported between moderate alcohol consumption and circulating TIMP-1 concentrations.41
Inflammation
Different inflammatory stimuli, such as TNFα and interleukin 1, as well as other factors, regulate the expression of MMP.60 In experimental studies it has been shown that C-reactive protein, an inflammatory biomarker of atherosclerotic risk, induces the expression of MMP-1 by macrophages, and of MMP-10 by endothelial cells, without affecting TIMP-1 concetrations.38,61 It has also been reported that circulating MMP-9 concentrations in patients with coronary heart disease, which are higher than those in healthy subjects, are directly associated with the concentrations of inflammation markers such as plasma C-reactive protein, interleukin 6, and fibrinogen. However, no differences in nor association with the MMP-2 levels have been reported.62 Similarly, in other clinical studies involving asymptomatic subjects, C-reactive protein have been associated with those of MMP-9 and 10, but not with those of MMP-2 or MMP-3.38 Recently, our group has shown that C-reactive protein induces the expression of MMP-1 and MMP-10 by human endothelial cells, and that asymptomatic subjects with a proinflammatory profile show elevated concentrations of MMP-10.38 Doxycycline, which reduces the concentration of C-reactive protein also reduces the activity of MMP-9.63 It has been proposed that the infection of macrophages or smooth muscle cells with Chlamydia pneumoniae can induce the production of MMP since a strong relationship has been seen between the presence of this bacterium and the immunodetection of MMP-9 in atherosclerotic plaques. However, no causal relationship has been established.64
PROGNOSTIC VALUE OF EXTRACELLULAR MATRIX BIOMARKERS
Quantification of the turnover of the ECM in cardiovascular tissues faces methodological difficulties. The different procedures available for evaluating fibrosis/degeneration in tissues (eg, endomyocardial biopsy, intravascular ultrasonography, etc) are, apart from being invasive, of limited use. Further, circulating ECM biomarkers are not specific for vascular tissue, and since MMP binds ECM components a local increase does not always correlate with a systemic increase. Currently, it is unsure which of the 40 possible ECM biomarkers has the best prognostic value. Therefore, when establishing criteria for the selection of biomarkers of interest and clinical applicability, certain factors should taken into account: a) it should be demonstrated that markers reflects the remodeling of the ECM, b) there should be evidence that high concentrations are found in patients with stable disease, c) the biomarker should be stable in plasma or serum, and d) the method should be standardized and show little variability. As well as showing good reproducibility, biomarkers should be helpful in arriving at a diagnosis, at a prognosis, and in the therapeutic monitoring of atherosclerosis.3,4
Several studies have reported an association between MMP and TIMP that predicts an adverse prognosis in a wide range of cardiovascular diseases.65-68 The most promising circulating biomarkers of ECM breakdown are MMP-9 (gelatinase B) and 10 (stromelysin 2). An increase in MMP-9 predicts a narrowing of the arterial lumen, restenosis after the positioning of a stent, and cardiovascular death in patients with coronary heart disease.69-72 It has also been related to the expansion and rupture of aortic aneurysms73,74 and increases the risk of hemorrhagic transformation in patients with ictus.75 These biomarkers may also offer prognostic information in the primary prevention setting. MMP-9 predicts ischemic heart disease, and/or high blood pressure in patients with no prior cardiovascular disease,76 and our group has recently shown that MMP-10 provides a marker of subclinical atherosclerosis; a correlation was seen between plasma MMP-10 levels and the thickness of the intima media of the carotid artery in a large group of patients with no history of cardiovascular disease.77
THE EXTRACELLULAR MATRIX AS A THERAPEUTIC TARGET IN ATHEROTHROMBOSIS
Modulation of pericellular proteolysis may be a good target for therapeutic intervention in the context of atherosclerosis.78 Perhaps the most representative example of the effect of therapeutic agents on vascular remodeling is provided by thrombolytic treatment. This stimulates the expression of MMP via plasmin, and promotes the degradation of collagen.79 Statins also reduce the concentration of MMP-9 and other MMP,80,81 as do angiotensin II type I receptor antagonists,82 while other agents with cardiovascular action (such as carvedilol and the thiazolidinediones) reduce concentration of MMP-1.83,84 Certain antibiotics, such as doxycycline and the tetracyclins, reduce the vascular and systemic expression of several MMP,63 while antioxidants reduce the expression of MMP-1.46
Several pharmaceutical companies are interested in developing synthetic inhibitors of MMP, although none are yet available for clinical use.85,86 Some experimental studies show that the inhibition of MMP via the use of TIMP may delay the progression of an atherosclerotic plaque.71,87 In addition, the inhibition of MMP in the advanced stages of atherosclerosis can protect against the development of unstable plaques, the formation of aneurysms and heart failure.88,89
CONCLUSIONS
Numerous studies have shown that the remodeling of the ECM of artery walls can be monitored by determining the circulating concentration of different MMP and TIMP. These molecules can be considered biomarkers of prognostic potential with respect to the recurrence of ischemic heart disease, the development of heart failure, and the formation of aneurysms in patients with clinical atherosclerosis. They may also be used in asymptomatic subjects with risk factors or in those with subclinical atherosclerosis. Prospective studies currently underway should allow us to clarify the diagnostic and prognostic potential of these biomarkers in vascular diseases, and to assess their prospects as new therapeutic targets in atherosclerosis.
Correspondence: Dr. J.A. Rodríguez.
Laboratorio de Aterosclerosis. Área de Ciencias Cardiovasculares. CIMA.
Avda. Pío XII 55. 31008 Pamplona. Navarra. España.
E-mail: josean@unav.es