INTRODUCTION
Spain has a history of maintaining registries on acute coronary syndrome (ACS),1-3 thus providing information on the characteristics of patients as well as disease progression and management. The MASCARA registry was similar to previous registries (PRIAMHO II1 and DESCARTES2), but included 2 special features: a) it covered the entire range of ACS: with ST-segment elevation (STEACS), without ST-segment elevation (NSTEACS), and where this characteristic could not be identified (nonclassifiable ACS); and b) it was developed soon after the publication of the Clinical Practice Guidelines on NSTEACS by the Spanish Society of Cardiology,4 and assesses their impact. Its aims were as follows: to determine the clinical characteristics, patient management strategies, and prognosis of ACS in Spain, and to compare, respectively, the effect—raw and adjusted by the main baseline prognostic factors—of primary percutaneous coronary intervention (PCI) with thrombolysis in patients with STEACS and that of an early intervention strategy (EIS) with that of non-early intervention in patients with NSTEACS.
METHODS
The study methodology has been previously described.5 This was a prospective study with the following characteristics: a) centers were randomly selected according to their level of care; b) it consecutively included patients who fulfilled the selection criteria and none of the exclusion criteria; and c) centralized telephone follow-up was conducted at 6 months. The patient selection criteria included the following 3 conditions: clinical manifestations compatible with ACS in the previous 24 h; admitted directly or by transfer to one of the hospitals included in the study (in any hospital area); and the diagnosis of ACS confirmed during admission. Any of the following 3 circumstances led to the diagnosis of ACS being confirmed: a) admission for anginal chest pain (or equivalent) with no significant or assessable changes in the electrocardiogram (ECG) but with elevated markers of myocardial necrosis or a positive screening test for ischemia during admission, or a history of known coronary heart disease; b) admission for anginal chest pain or equivalent and changes on ECG compatible with myocardial ischemia; and c) absence of chest pain, but with elevated markers and changes on ECG compatible with ischemia or positive screening test for ischemia during admission. The exclusion criteria included the following: not possible to complete follow-up; myocardial ischemia clearly triggered by an extracardiac cause; extracardiac diseases concomitant with a vital prognosis of less than 12 months; and absence of informed consent. Except for the second criterion, these exclusion criteria refer to the telephone follow-up, not to the baseline data.
STEACS was defined as ST-segment elevation 31 mm in at least 2 leads for more than 20 min. Nonclassifiable ACS was defined as pacemaker rhythm, advanced left bundle branch block, or Wolff-Parkinson-White syndrome on ECG. NSTEACS was diagnosed in the patients who fulfilled diagnostic criteria for ACS without belonging to the other 2 categories.
In line with the most recent recommendations of the European Society of Cardiology, EIS was defined as coronary angiography in the first 72 h of the index event in patients admitted for NSTEACS6; and primary PCI was defined as coronary angiography during the first 12 h of hospital admission in the absence of previous thrombolysis in patients diagnosed with STEACS.
Researchers from each center identified the patients, requested their informed consent, and performed an ECG. Subsequently, trained monitors periodically visited the hospital and completed an extensive questionnaire on baseline characteristics and disease progression. The questionnaires used in the study, together with the definitions of the variables, can be consulted at: http://www.uesca.net/
The primary outcome variables were as follows: a) hospital mortality; b) mortality from any cause at 6 months; and c) mortality or readmission for ACS during the first 6 months following the index event.
The recruitment period was September 2004 to June 2005.
Quality Control
The comprehensiveness of the inclusion process was submitted to quality control between 2005 and 2006 by means of the following:
1. All the hospitals, except for 7 centers whose inclusion process was already clearly defective, were asked to provide their discharge lists (codes 410, 411, 413, and 414 in the Conjunto Mínimo Básico de Datos [Set of Minimum Basic Data]).
2. The data center and the coordinating center (Instituto Municipal de Investigaciones Médicas de Barcelona and Unidad de Epidemiología del Servicio de Cardiología del Hospital Vall d'Hebron), excluded erroneous codings and the discharge lists were cross-checked with the databases of the patients included in the study up to that point. This provided a list of potentially suitable patients during the study period who had not been included.
3. Subsequently, candidates suitable for inclusion were identified among the group that had not been included, by checking on a case-by-case basis (or based on the discharge report) in every center. This was performed by a team of nurses trained for this task or by local researchers who agreed to do it. The patients who were located in this way were included retrospectively. Although telephone follow-up could not be conducted due to the lack of informed consent from these patients, follow-up was performed using each center's computer registries to identify new admissions and their causes based on the medical record. A cohort was thus obtained with a strong guarantee of having undergone comprehensive and unbiased inclusion (gold standard) in 18 centers that accepted this approach to quality control (4889 records).
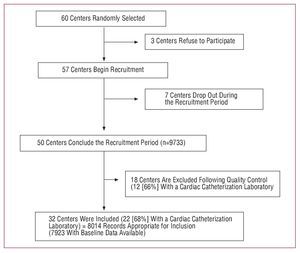
In a second quality control phase, this reference cohort was compared to the patients included in each center that had not followed the quality control procedure (33 centers; 4219 records). In this subanalysis, centers were identified whose patients presented a lower mortality rate and who occasionally presented a risk factor distribution that was quite different from that of the reference group. For example, hospital mortality due to STEACS in one of the tertiary centers was 2% (95% confidence interval [CI], 0.2-4.3), whereas that of the reference group was 7.5% (95% CI, 6.1-8.6), which indicated an inclusion bias due to not including the most seriously ill patients. In an attempt at minimizing this bias, the scientific committee defined some criteria in order to exclude from the analysis the centers in which the suspicion of biased inclusion was high. Specifically, centers were excluded from the present analysis where the researcher had aknowledged the inclusion of patients that was clearly incorrect or whose inclusion rate was less than 50% of that expected according to the level of care provided by the center and, in addition, where the 95% CI of its mortality rate for each type of ACS did not include the mean value of the reference centers. Thus, 18 of the 50 centers that had concluded recruitment and in which, following the criteria described, inclusion had been clearly incorrectly performed were excluded from the present analysis (Figure 1). As part of the data inclusion process and database debugging, each registry was thoroughly checked, and the researcher or medical record consulted in case of inconsistency.
Figure 1. Selection history of centers included in the study.
Statistical Analysis
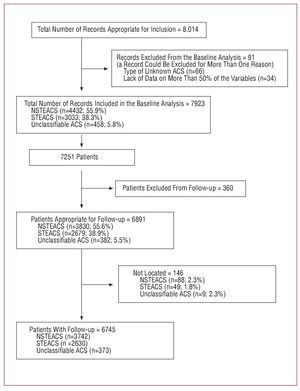
A total of 7923 records were analyzed regarding baseline characteristics and hospital management corresponding to 7251 patients (several patients were admitted more than once). The raw and adjusted mortality rates were analyzed in these 7251 patients, as well as the survival rates where the output variables "death" and "death or readmission" at 6 months were assessed.
Discrete variables were expressed as percentages, based on all the valid data. A confidence interval of 95% was estimated. Continuous variables were expressed as mean (standard deviation) or as medians (interquartile range) according to the underlying distribution whether normal or otherwise.
Baseline data on the different types of ACS were compared using the c2 test for discrete variables and the Kruskal-Wallis test for continuous variables (given the deviation from the assumptions of normality of the underlying distribution).
Survival analyses were performed using the Kaplan-Meier method. The survival curves and survival free from readmission at 6 months were compared using the log-rank test.
The adjusted effects of EIS in NSTEACS and of the type of reperfusion in STEACS were analyzed using the Cox proportional risks model for mortality and mortality or readmission for ACS at 6 months in 2 populations: in all patients, and in those discharged alive during the index event. First, a predictive model was constructed based on the variables that had a significant association with mortality in the bivariate analysis and all those which were considered clinically relevant. Specifically, in NSTEACS, the following variables were introduced in the initial model: age, sex, hypertension, diabetes, dyslipidemia, smoking, peripheral arterial disease, history of infarction, elevated necrosis markers, ST-segment deviation in the baseline ECG >0.5 mm, initial systolic blood pressure, initial heart rate, creatinine concentrations >1.4 mg/dL, treatment with beta-blockers in the first 24 h, administration of glycoprotein IIb/IIIa antagonists, and Killip class. In the case of STEACS, however, the variable infarction location (anterior vs other) was included, but not elevated necrosis markers or ST-segment deviation. Predictors of mortality at 6 months were analyzed using the stepwise backward elimination procedure (with an input P value <.05 and an output P value >.1). Based on the resulting model the variable "reperfusion strategy" was introduced in NSTEACS and "type of reperfusion" in STEACS. Subsequently, the remaining variables that had not fulfilled the automatic criteria for the modeling system were assessed and introduced in the event that they led to a clinically relevant change in the adjusted effect of the main variable. In the models for the patients who survived the index event, both in NSTEACS and STEACS, treatments at discharge were also assessed. The assumed proportion of the proportional risks was verified in the categories of the variables introduced into the models.
The analyses were performed using the statistical packages SPSS 13.0 and SAS 9.
RESULTS
A total of 60 centers were randomly invited to participate, of which 3 declined. Of the 57 centers selected, 50 recruited 9733 patients; 18 centers were excluded after the quality control process (1719 patients) after verifying that their total recruitment was less than that required by the study. Finally, 32 centers remained that included 8014 records, of which 7923 were valid and corresponded to 7251 patients, on which the analysis of the hospital outcomes (Figures 1 and 2) was based.
Figure 2. History of patient inclusion.
Table 1 presents the baseline data of the patients according to the type of ACS. In the nonclassifiable ACS group, the patients were older, with more women and with general characteristics of greater clinical severity. It highlights the high rate of diabetes and that of atherosclerosis in other locations. The patients with STEACS had less history of coronary heart disease and lower rates of previous treatments.
Table 2 presents the clinical manifestations, management, and outcomes in the first 48 h after the index event. The following factors are highlighted: the median of almost 2 hours between symptom onset and arrival in the emergency department; and consistent with the data presented in Table 1, the greatest percentage of Killip class >I and creatinine concentrations >1.4 mg/dL was found among the patients with nonclassifiable ACS. Approximately 68% of the patients with STEACS received reperfusion therapy during the acute phase and thrombolysis (with or without rescue PCI) was the most frequent treatment. Primary PCI was performed in 24.7% (95% CI, 23.2-26.3) of the admissions and rescue PCI in 10.7% (95% CI, 9.8-12). The median door-to-needle time was 45 min (semiquartile range, 25-75), and median door-to-balloon time, 97 min (semiquartile range, 60-203).
Table 3 presents the angiographic data, management, and outcomes corresponding to the entire admission period. Two-thirds of the patients underwent echocardiographic study, whereas few screening tests for ischemia were performed. The greatest percentage of left main coronary artery disease and significant 3-vessel disease was observed in nonclassifiable ACS. The table highlights the different percutaneous coronary revascularization rates in the various types of ACS; this was relatively high in STEACS and low (19.4%) in the patients with a worse prognosis (unclassifiable ACS), while the use of surgery during admission or programmed surgery was 5%. The rate of non-cerebral hemorrhagic stroke was low but nontrivial, especially in patients older than 75 years (4%; 95% CI, 3.3-5.1).
Mean total hospital mortality was 5.7% (NSTEACS, 3.9%; STEACS, 7.6%; and unclassifiable ACS, 8.8%).
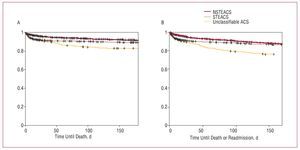
Data on follow-up after discharge were obtained for 6745 patients (90% of appropriate patients). Figure 3 shows the total survival curves and survival free from readmission for ACS at 6 months. Although survival at 6 months in NSTEACS and STEACS were similar, 90.3% (95% CI, 89.4-91.3%) and 88.2% (95% CI, 87-89.5), respectively, survival in nonclassifiable ACS was clearly lower: 83.9% (95% CI, 86-87.9) (P<.0001). The survival free from readmission rates for ACS in NSTEACS and STEACS were 87.2% (95% CI, 86-88.3) and 86.4% (95% CI, 85.1-87.8), respectively, whereas in nonclassifiable ACS it was 75.2% (95% CI, 70.5-80) (P<.0001).
Figure 3. Survival curves at 6 months from the index event according to the type of ACS. A: total survival. B: survival free from readmission.
Treatment rates at discharge (Table 4) indicate a strong increase in the use of drugs compared to previous registries and that had already been observed regarding the drugs and procedures used in the initial phase (Table 5).
Early Intervention Strategy in NSTEACS
Data on the type of strategy used (EIS vs non-EIS) were obtained for 4046 records (91%). An EIS was performed in 795 (19.6%) events. Table 6 shows the baseline characteristics of the NSTEACS events in which an EIS was used versus the remaining patients. Those who underwent EIS were, in general, at lower risk than those who did not: more young people, with less prevalence of kidney failure and diabetes, and with an initial lower Killip class. However, they were more often admitted to centers with a cardiac catheterization laboratory available and elevated necrosis markers were greater than in those who did not undergo EIS.
Raw hospital mortality, based on 3473 patients with valid data, was somewhat greater in the EIS group, although the difference was not significant. However, this trend reversed over time, such that at 6 months mortality in the EIS group (based on 3211 patients with valid data and complete follow-up) was significantly less than in the remaining patients.
Table 7 shows the effect of EIS on mortality at 6 months adjusted by the most relevant predictive variables, both in the total population and in the patients who survived the index event. No association was observed between EIS and outcome in the total population. However, in the patients who survived the index event, a clinically relevant association was observed in EIS, although this did not reach statistical significance. Similar outcomes were found regarding the variable "death or readmission for ACS" at 6 months, both in the total population (hazard ratio [HR] = 0.92; 95% CI, 0.65-1.3) as well as in those who survived the index event (HR=0.65; 95% CI, 0.39-1.06; P=0.086). Finally, the analysis was repeated excluding the patients who died in the initial hours (0.6%) and those who did not undergo catheterization who were initially assigned to the non-EIS group, but without any significant changes being observed.
Reperfusion in STEACS
Valid data on the type of reperfusion performed were obtained for 3000 (98.8%) records. Table 8 shows the baseline characteristics of patients with STEACS who underwent thrombolysis (with or without rescue angioplasty) compared to those undergoing primary PCI and those not undergoing any type of reperfusion who, in general, were at greater risk than those undergoing some reperfusion strategy: more advanced age, higher prevalence of women, diabetes, kidney failure, and a more severe Killip class. In contrast, the patients treated with thrombolysis had a lower risk profile than the other patients.
Raw hospital mortality, based on 2628 patients with valid data, was greater in the patients who did not undergo any type of strategy than in those who underwent thrombolysis or primary PCI. Hospital mortality was greater in the latter group than in those who underwent thrombolysis. Differences in mortality among patients, depending on whether reperfusion was performed or not, increased over time. This was not the case regarding differences in mortality between thrombolysis and the primary PCI, as these decreased at 6 months.
Angioplasty was performed after thrombolysis in 11.3% of all the patients. Mortality in this subgroup was very similar to that in patients who underwent thrombolysis only, including both hospital mortality (6.1%; 95% CI, 3.6-9.4) and at 6 months (10.5%; 95% CI, 7-15). Multivariate analysis of the total of patients with valid data showed no association of thrombolysis or primary PCI with mortality at 6 months. However, in the patients who survived the index event, a relevant and statistically significant association was found in those undergoing primary PCI compared to those not undergoing reperfusion (Table 9). The effect of thrombolysis on prognosis, compared to no reperfusion, was not conclusive, and neither was the effect of primary PCI compared to thrombolysis. Similar outcomes were obtained with the variable "death or readmission for ACS" at 6 months, both in the total population (thrombolysis: HR=1; 95% CI, 0.77-1.4; primary PCI: HR=1; 95% CI, 0.73-1.45) and in patients surviving the index event (thrombolysis: HR=0.7; 95% CI, 0.45-1 [P=.1]; primary PCI: 0.53; 95% CI, 0.3-0.9 [P=.03]). As an additional analysis, patients who subsequently underwent follow-up rescue angioplasty were excluded from the thrombolysis group, although no association was found between thrombolysis and mortality in the total population (HR=1.1; 95% CI, 0.83-1.6) or between thrombolysis and those who survived the index event (HR=0.82; 95% CI, 0.49-1.3).
DISCUSSION
The MASCARA study offers an overview of the clinical profile, management and outcomes of patients admitted for ACS in Spanish hospitals 1 year after the Sociedad Española de Cardiología clinical practice guidelines were implemented.4 The results indicate that, compared to previous registries, there was a striking increase in the use of recommended drugs and interventions in 2004-2005. However, as assessed in the total population and adjusting for the main baseline prognostic factors, no association was observed between primary PCI in STEACS and EIS in NSTEACS regarding mortality and mortality or readmission for ACS at 6 months. This was not the case in the patients who survived the index event, where there was an association, although this was not conclusive in the EIS.
General Management of ACS
The results of the study are consistent with those observed in other registries.7,8 Furthermore, the MASCARA registry showed a striking increase in the use of drugs and procedures recommended by the clinical practice guidelines compared to the PRIAMHO II and DESCARTES registries (Table 5). These findings had already been demonstrated in previous studies.9,10
Nevertheless, comparisons between registries have serious limitations given the differences between the corresponding populations and selection criteria. The recent change in the definition of ACS has to be added in the case of the MASCARA study. Thus, comparing its results to those of other Spanish registries is hazardous, especially regarding the output variables. For example, the DESCARTES registry included 18% of patients without confirmed ACS,2 whereas the verification of this diagnosis was a requirement in the MASCARA study.5 This could explain the differences in mortality between the 2 studies. In the case of STEACS, comparisons with the PRIAMHO II study is even more problematic, since different selection criteria and definitions were used,1,11 and it only included patients admitted to coronary care units.
Early Intervention Strategy in NSTEACS
The EIS was conducted in a minority of the patients (19.6%) and generally in those with a lower baseline risk, in contrast to what is recommended.6 This finding, which has already been shown in other registries,8 highlights the difficulties involved in implementing the clinical practice guidelines.
Several factors related to patient management (availability of a cardiac catheterization laboratory, logistic elements, care routines, etc) may have contributed to this. Research into these factors was not an aim of the MASCARA registry, and they probably require deeper study. In any case, the results indicate a possible patient management problem in some Spanish centers entailing poor consequences regarding prognosis.
In the adjusted analysis, no association was observed between EIS and the output variables in the total population. The absence of such an association could be at least partially related to the selection of patients for EIS. The fact that, in the sample analyzed, most of the factors most strongly associated with the baseline risk of death in hospital and at 6 months were more prevalent in those not undergoing an EIS could explain the lack of association of this with prognosis after statistical adjustment. It could also explain the difference in mortality observed between the 2 groups during follow-up, where a markedly higher increase in mortality after hospital discharge was observed in the patients who initially did not undergo an EIS (8.9%) compared to those who did (4.6%). It is worth considering whether different outcomes would have been obtained if the patients at greater baseline risk had followed an EIS. Another factor to take into account, when attempting to explain the lack of an association between the EIS with prognosis, is the revascularization rate among patients who did not follow an EIS (33.1%) (Table 6). This was much higher than that found in the FRISC II study (9%)12 which demonstrated the benefit of an EIS, whereas it was closer to that found in the ICTUS study (40%)13 which did not demonstrate any such benefit. Finally, we should take into account the fact that the greatest benefit of an EIS is more clearly demonstrated in the long term, whereas in the MASCARA study follow-up could only be only performed at 6 months.
The observation of an association between the EIS and prognosis in the analysis, excluding the patients who died during the index event, is difficult to interpret. It could be hypothesized that, as mentioned, factors associated with patient management relating to the initial implementation of a technique requiring an extended learning period may have led to hospital outcomes that were less than desirable, as indicated by raw mortality rates greater than those found in the early non-intervention strategy. Perhaps the patients who underwent an EIS and survived the index event were those in whom this was carried out optimally and, thus, were those in whom the potential short-term benefit of the EIS was concentrated. In any case, other studies have shown high mortality in the acute phase of patients undergoing revascularization, although the long-term prognosis was favorable in these patients.12
Reperfusion in STEACS
As in the case of NSTEACS, the total risk among patients not undergoing coronary reperfusion was greater than among those where this was performed, although the differences were less marked in relation to the group treated by primary PCI.
We note that hospital mortality was greater among patients undergoing primary PCI compared to thrombolysis, and this was probably related to a higher prevalence of factors strongly related to prognosis in this group. Nevertheless, this difference in mortality decreased over time and lost statistical significance at 6 months.
In the adjusted analysis, we highlight the lack of an association between reperfusion (thrombolysis or primary PCI) with the output variables in the total population. This finding was reversed when the patients who died during the index event were excluded from the analysis, which again suggests that there could be factors associated with patient management that could not be considered in the analysis, perhaps related to the in-hospital prognosis and that could act as confounders. For example, the door-to-needle and door-to-balloon reperfusion times were only obtained in a low number of patients, and thus could not be introduced as adjustment variables in the analysis. In any case, the available times were longer than those recommended in the guidelines and it is not likely that the unrecorded times were any lower. If this variable had been available in a sufficient number of patients, it may have been possible to identify the subgroup in which an optimal strategy was followed and the benefit of reperfusion possibly concentrated.
As an indirect approach to identifying this subgroup, the patients who died during the index event were excluded from the analysis (as well as from the NSTEACS analysis). A benefit from thrombolysis was then observed, although this was not conclusive—perhaps due to the lack of statistical power—and a clinically relevant and statistically significant benefit from primary PCI. Following the same strategy, a not conclusive benefit of primary PCI compared to thrombolysis was also observed.
Internal and External Validity of the Study Results
In order to ensure the internal validity of our results, only the centers for which comprehensive and unbiased inclusion (70%) was confirmed directly and indirectly were included.
Regarding external validity, we consider that the 32 centers finally included in this analysis suitably reflect the true nature of patient management in Spain, since the centers proposed for inclusion were randomly selected and the 18 excluded centers had the same proportion of centers with a cardiac catheterization unit, thus indicating that their exclusion did not introduce any relevant bias. The exclusion strategy regarding the centers with deficient involvement is not exclusive to the present registry. Other observational studies have attained similar rates of involvement, also using quality criteria, and that are not very different from the ones in our study.2
It should be taken into account that the MASCARA study investigated effectiveness and not efficacy, which means that it cannot be inferred from the results that the EIS or primary PCI are not beneficial when they are applied under certain conditions. Furthermore, any causal inference based on this type of design is at least risky. However, after adjusting for the baseline prognostic factors, as no association was observed between the interventions under study and the results, it could be hypothesized that this may have been due to the existence of numerous variables related to patient management and that were not an objective of the study.
The results of a registry should be interpreted within a specific temporal framework. It is to be expected that the results obtained in the present registry do not fully correspond to the current situation. However, they demonstrate the difficulty involved in transferring the results of clinical trials to daily clinical practice, and even more so in the case of implementing therapeutic strategies within a critical period that involves complex logistics and whose end result depends on multiple technical and organizational factors.
Limitations
As the present study was observational, the existence of an inclusion bias cannot be completely ruled out. On the other hand, as mentioned, confounding variables could exist related to patient management that were not considered, since assessing these did not form part of the aims of the study. However, this possibility does not invalidate the conclusions of the study, taking into account its aims.
The value of the door-to-needle and door-to-balloon times is limited, since only a very low number of these were obtained, and thus this variable should be interpreted with caution. Similarly, neither could periprocedural ACS be recorded, which is a variable of interest when assessing the benefit/nonbenefit of intervention.
In the case of NSTEACS, no information was available on the number of patients who had undergone a screening test for ischemia ("conservative" strategy) or not (non-early "intervention" strategy) prior to cardiac catheterization, but who did not belong to the EIS group.
CONCLUSIONS
Following the dissemination of the clinical practice guidelines, the MASCARA study on ACS patient management in Spain, interpreted together with previous registries, indicates that in 2004-2005 new drugs and invasive procedures were quickly adopted, although no association was observed between an EIS in NSTEACS or reperfusion in STEACS in relation to prognosis at 6 months in the total population. These findings highlight the existence of factors related to patient management that prevent these strategies from being associated with prognosis, and perhaps the key factor is the failure to appropriately adapt these strategies to the baseline risk of the patients.
ANNEX. Researchers of the MASCARA Study
Dr Radován and Dr Maulén (Hospital de Campdevanol; Girona). Dr Ortiz de Murua, Dr Marcos, and Dr Arribas (Hospital Virgen de la Concha; Zamora). Dr Laperal and Dr Casado (Hospital de Calatayud; Zaragoza). Dr Bisbe (Hospital Sant Jaume de Olot; Girona). Dr Bartomeu, Dr Carrillo, and Asunción Mateu (Hospital Universitario Sant Joan d'Alacant). Dr Gutiérrez and Dr Benítez (Hospital Virgen del Puerto; Plasencia). Dr de Miguel, Dr Martínez, and Dr Soriano (Hospital de Terrassa). Dr Arias e Isabel Gómez (Hospital de Montecelo; Pontevedra). Dr Ortega and Dr Molina (Hospital Sta, María del Rossell; Cartagena). Dr Herreros and Dr Azcárate (Clínica Universitaria de Navara). Dr Worner, Dr Piqué, and Purificación Cascant (Hospital Arnau de Vilanova; Lleida). Dr Salvador and Dr Aguar (Clínica Dr Pesset; Valencia). Dr Arós and Dr Sanz (Hospital de Txagorritxu; Vitoria). Dr Velasco and Dr Belchi (Hospital Gral, Universitario de Valencia). Dr Pagola and M Amparo Pérez (Hospital Ciudad de Jaén). Dr Sogorb and Dr Oliver (Hospital Gral, Universitario de Alicante). Teresa Martorell, Dr Bórqued and Dr Verbal (Hospital Clínic i Provincial; Barcelona). Dr Esplugues, Dr Ribas, and Cristina Carvajal (Ciudad Sanitaria de Bellvitge; Barcelona). Dr Martín and Dr Pabón (Hospital Universitario de Salamanca). Dr Froufe, Dr León, and Dr Montes (Hospital de Cruces; Bilbao). Dr Poveda, Dr Ruiz, and Marta Calvo (Hospital Universitario Marqués de Valdecilla; Santander). Dr Alcalde, Dr Alguersuari, Dr Otaegui, and Purificación Cascant (Hospital Vall d'Hebron; Barcelona). Dr Juan, Dr Barrio, and Dr Estévez (Hospital Universitario Gregorio Marañón; Madrid). Dr Moreno and Dr Martín (Hospital San Cecilio; Granada). Dr Fernández Avilés and Dr Sánchez (Hospital Clínico Universitario de Valladolid). Dr Bruguera, Dr Soriano, and Dr Recasens (Hospital del Mar; Barcelona). Dr Abizanda and Dr Micó (Hospital Gral de Castellón). Dr Huelmos (Fundación hospital de Alcorcón). Dr Ortigosa and Dr Silva (Clínica Puerta de Hierro; Madrid). Dr Bardají, Dr Serrano, and Purificación Cascant (Hospital Joan XXIII; Tarragona). Dr Sala, Isabel Ramió, and Ruth Martí (Hospital Josep Trueta; Girona). Dr Montón (Hospital Gral, Yagüe; Burgos).
ABBREVIATIONS
ACS: acute coronary syndrome
ECG: electrocardiogram
EIS: early intervention strategy
NSTEACS: non-ST-segment elevation acute coronary syndrome
PCI: percutaneous coronary intervention STEACS: ST-segment elevation acute coronary syndromeSEE ARTICLE ON PAGES 793-6
Financial support: RECAVA, FIS (PI04/1408, PI04/1583) and an open grant from Bristol-Myers-Squibb.
The researchers and hospitals participating in the MASCARA study are listed in the ANNEX.
Correspondence:
Dr. G. Permanyer Miralda.
Unidad de Epidemiología. Servicio de Cardiología. Hospital Vall d'Hebron. Pg. de la Vall d'Hebron, 119-129. 08035 Barcelona. España.
E-mail: gpermany@gmail.com
Received October 25, 2007.
Accepted for publication May 6, 2008.