In recent years, implantation of cardiac resynchronization therapy devices has significantly increased. The benefits of this therapy are directly related to the maintenance of continuous biventricular pacing. This study analyzed the incidence, causes, and outcomes of loss of continuous biventricular pacing, and the approach adopted.
MethodsWe analyzed the clinical and follow-up data of a series of consecutive patients from a single center who underwent implantation of a cardiac resynchronization therapy device.
ResultsThe study included 136 patients. During a mean follow-up of 33.4 months, loss of continuous biventricular pacing occurred in 45 patients (33%). The most common causes included atrial tachyarrhythmias (21.3%), lead macrodislodgement (18%), and loss of left ventricular capture (13.1%). In most patients (88.5%), loss of continuous biventricular pacing was transient and correctable, and occurred earlier in the follow-up when the cause was lead macrodislodgement, oversensing, or extracardiac stimulation. There were no significant differences in mortality between patients with and without loss of continuous biventricular pacing (P=.88).
ConclusionsDespite technical advances in cardiac resynchronization therapy, loss of continuous biventricular pacing is common; however, this loss can usually be corrected. In most patients, continuous biventricular pacing can be ensured by close monitoring and follow-up and a proactive approach.
Keywords
.
INTRODUCTIONHeart failure (HF) has become a worldwide pandemic. Because of its high prevalence, this disease represents the most important health problem in contemporary cardiovascular medicine. In Spain, as in other western countries, at least 2% of individuals over the age of 40 years have HF and the incidence of this disease increases progressively with age, reaching 6% to 10% in persons over 65 years of age.1,2 A number of randomized multicenter studies have demonstrated that cardiac resynchronization therapy (CRT) can significantly reduce morbidity and mortality rates in subgroups of patients with HF associated with left ventricular (LV) systolic dysfunction and intraventricular conduction disturbances.3–5 Due to the expansion of the indications for CRT,6–11 implantation of CRT devices has substantially increased in recent years.12 However, despite the potential benefits of this therapy, there are several important factors that limit its use: a) approximately one third of treated patients do not respond favorably to CRT, and some do not even tolerate biventricular pacing, despite careful optimization of the parameters13,14; b) to obtain the maximum benefit, the highest possible percentage of biventricular pacing must be achieved,15-16 and c) in patients with a CRT device, there are a number of circumstances that can produce the transient or permanent loss of biventricular pacing, limiting the potential long-term benefit of this therapy.
There is little information in the literature on the incidence and possible causes of CRT loss in studies involving series of patients.17 This report presents the experience of a single center in an effort to determine this incidence and identify the causes of CRT loss, the approach adopted, and the results of the measures taken.
METHODSPatientsWe performed a prospective and retrospective observational study in which we analyzed the clinical and follow-up data of a series of consecutive patients who underwent successful implantation of a CRT device in a single center. The devices were programmed according to the estimates of the implanting physicians, who individualized the programming for each patient according to his or her personal characteristics, and in such a way as to ensure continuous biventricular pacing (adjustment of the pacing mode, upper and lower stimulation frequency limits, atrioventricular intervals, algorithms for ventricular rate smoothing and for resynchronization in premature ventricular contractions, etc.). The clinical and technical data were obtained from the medical record, the implantation procedure report, and device follow-up. For all patients, we collected general demographic data, including age and sex, medication, functional class, QRS complex width, type of conduction disturbance, baseline rhythm, and previous history of atrial arrhythmias and the type, as well as data on the indication for implantation, the implementation of appropriate and inappropriate therapies during follow-up, the clinical response to CRT, the time elapsed between implantation and documentation of the loss of biventricular stimulation, when relevant, and between implantation and the last recorded follow-up visit. Periodic follow-up visits and device interrogation were performed at maximum intervals of 6 months.
DefinitionsLoss of Continuous Biventricular PacingBiventricular pacing of less than 90% of the beats, identified in any device interrogation when compared with the previous examination, and always with an interval of at least 3 months between visits. More than 1 cause could be identified in a patient, but a given cause was considered only once in each patient. The loss was considered to be permanent when continuous biventricular pacing greater than 90% could not be restored, despite the measures adopted after identifying the problem. Otherwise, the loss was considered to be transient.
Ventricular Lead DislodgementLoss of ventricular capture due to an increase in the pacing threshold because of lead macrodislodgement demonstrated by radiological studies.
Failure of Ventricular CaptureLoss of ventricular capture due to an increase in the pacing threshold produced by causes other than ventricular lead macrodislodgement.
Responder to Cardiac Resynchronization TherapyAny patient whose HF symptoms improved after device implantation, expressed as an improvement in the New York Heart Association (NYHA) functional class.
Statistical AnalysisData analysis was performed using the SPSS statistical software package (version 20.0). Continuous variables are expressed as mean (standard deviation); the categorical variables, as absolute values and percentages. For the comparison of continuous variables, the Student t test or Mann-Whitney U test was employed. The categorical variables were compared using contingency tables and application of the chi-square test or Fisher's exact test. The study of the time to documentation of the loss of continuous biventricular pacing was carried out according to the Kaplan-Meier method. For the search for independent predictors of the loss of continuous biventricular pacing, we used a binary logistic model, taking as the dependent variable the probability of loss of continuous biventricular pacing versus the remaining variables employed in the analysis. Statistical significance was set at P<.05.
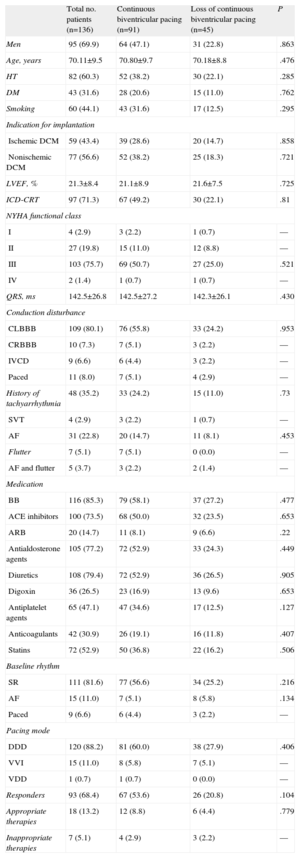
RESULTSWe studied a total of 136 consecutive patients who underwent CRT device implantation. The general characteristics of the patients are shown in Table 1. Briefly, over two thirds were men, with a mean age of 70 years. The most frequent indication for CRT was nonischemic dilated cardiomyopathy (56.6%) and the LV ejection fraction was markedly depressed. In 97 of the 136 patients, the CRT device was combined with an implantable cardioverter-defibrillator. Three fourths of the patients were in NYHA functional class III, and the most prevalent conduction disturbance in the study population was left bundle branch block. Importantly, slightly more than one third of the patients had a history of atrial tachyarrhythmias, but only a third of those patients had them at the time of implantation. The predominant programmed pacing mode was DDD (88.2%). Approximately 70% of the patients were considered to be CRT responders according to the criterion based on improvement in NYHA functional class.
General Characteristics of the Patients
| Total no. patients (n=136) | Continuous biventricular pacing (n=91) | Loss of continuous biventricular pacing (n=45) | P | |
| Men | 95 (69.9) | 64 (47.1) | 31 (22.8) | .863 |
| Age, years | 70.11±9.5 | 70.80±9.7 | 70.18±8.8 | .476 |
| HT | 82 (60.3) | 52 (38.2) | 30 (22.1) | .285 |
| DM | 43 (31.6) | 28 (20.6) | 15 (11.0) | .762 |
| Smoking | 60 (44.1) | 43 (31.6) | 17 (12.5) | .295 |
| Indication for implantation | ||||
| Ischemic DCM | 59 (43.4) | 39 (28.6) | 20 (14.7) | .858 |
| Nonischemic DCM | 77 (56.6) | 52 (38.2) | 25 (18.3) | .721 |
| LVEF, % | 21.3±8.4 | 21.1±8.9 | 21.6±7.5 | .725 |
| ICD-CRT | 97 (71.3) | 67 (49.2) | 30 (22.1) | .81 |
| NYHA functional class | ||||
| I | 4 (2.9) | 3 (2.2) | 1 (0.7) | — |
| II | 27 (19.8) | 15 (11.0) | 12 (8.8) | — |
| III | 103 (75.7) | 69 (50.7) | 27 (25.0) | .521 |
| IV | 2 (1.4) | 1 (0.7) | 1 (0.7) | — |
| QRS, ms | 142.5±26.8 | 142.5±27.2 | 142.3±26.1 | .430 |
| Conduction disturbance | ||||
| CLBBB | 109 (80.1) | 76 (55.8) | 33 (24.2) | .953 |
| CRBBB | 10 (7.3) | 7 (5.1) | 3 (2.2) | — |
| IVCD | 9 (6.6) | 6 (4.4) | 3 (2.2) | — |
| Paced | 11 (8.0) | 7 (5.1) | 4 (2.9) | — |
| History of tachyarrhythmia | 48 (35.2) | 33 (24.2) | 15 (11.0) | .73 |
| SVT | 4 (2.9) | 3 (2.2) | 1 (0.7) | — |
| AF | 31 (22.8) | 20 (14.7) | 11 (8.1) | .453 |
| Flutter | 7 (5.1) | 7 (5.1) | 0 (0.0) | — |
| AF and flutter | 5 (3.7) | 3 (2.2) | 2 (1.4) | — |
| Medication | ||||
| BB | 116 (85.3) | 79 (58.1) | 37 (27.2) | .477 |
| ACE inhibitors | 100 (73.5) | 68 (50.0) | 32 (23.5) | .653 |
| ARB | 20 (14.7) | 11 (8.1) | 9 (6.6) | .22 |
| Antialdosterone agents | 105 (77.2) | 72 (52.9) | 33 (24.3) | .449 |
| Diuretics | 108 (79.4) | 72 (52.9) | 36 (26.5) | .905 |
| Digoxin | 36 (26.5) | 23 (16.9) | 13 (9.6) | .653 |
| Antiplatelet agents | 65 (47.1) | 47 (34.6) | 17 (12.5) | .127 |
| Anticoagulants | 42 (30.9) | 26 (19.1) | 16 (11.8) | .407 |
| Statins | 72 (52.9) | 50 (36.8) | 22 (16.2) | .506 |
| Baseline rhythm | ||||
| SR | 111 (81.6) | 77 (56.6) | 34 (25.2) | .216 |
| AF | 15 (11.0) | 7 (5.1) | 8 (5.8) | .134 |
| Paced | 9 (6.6) | 6 (4.4) | 3 (2.2) | — |
| Pacing mode | ||||
| DDD | 120 (88.2) | 81 (60.0) | 38 (27.9) | .406 |
| VVI | 15 (11.0) | 8 (5.8) | 7 (5.1) | — |
| VDD | 1 (0.7) | 1 (0.7) | 0 (0.0) | — |
| Responders | 93 (68.4) | 67 (53.6) | 26 (20.8) | .104 |
| Appropriate therapies | 18 (13.2) | 12 (8.8) | 6 (4.4) | .779 |
| Inappropriate therapies | 7 (5.1) | 4 (2.9) | 3 (2.2) | — |
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin receptor blocker; BB, beta-blockers; CLBBB, complete left bundle branch block; CRBBB, complete right bundle branch block; DCM, dilated cardiomyopathy; DM, diabetes mellitus; HT, hypertension; ICD-CRT, implantable cardioverter-defibrillator with cardiac resynchronization therapy; IVCD, intraventricular conduction disturbance; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SR, sinus rhythm; SVT, supraventricular tachycardia.
Data are expressed as mean±standard deviation or no. (%).
During a mean follow-up period of 33.4 months, 25 patients (18.4%) died, due to HF in 69% and to sudden arrhythmic death in 15.4%; only 18 patients (13.2%) received appropriate therapy with the device and 7 patients (5.1%) received inappropriate therapy, mainly due to supraventricular arrhythmias incorrectly classified by the device.
Loss of Cardiac Resynchronization TherapyDuring follow-up, 45 patients (33.1%) experienced transient or permanent loss of continuous biventricular pacing, with an estimated annual incidence of 12.5%. We observed no significant differences in the general patient characteristics between those with loss of continuous biventricular pacing and those without (Table 1). In 16 patients (11.7%), we identified more than 1 cause of the loss during follow-up; in all, 61 causes were recorded in the 45 patients. The causes of the CRT loss identified and the numbers of patients affected are shown in Table 2. The most common causes of the CRT loss were atrial tachyarrhythmias, LV lead dislodgement, and failure of LV capture, which constituted slightly more than half of the overall events (32 of 61). Continuous biventricular pacing could be restored, that is, the loss was transient, in most patients (in 88% when considering the overall causes and in 84.44% of the patients). The loss of continuous biventricular pacing was permanent in only 7 patients: the most common cause was atrial tachyarrhythmias (3 of the 7 cases) followed by LV lead dislodgement, failure of LV capture, and recurrent ventricular arrhythmias were the causes in 1 patient each. The mean time to the documentation of loss of continuous biventricular pacing varied depending on the identified cause, and occurred earlier in cases of extracardiac stimulation, lead dislodgement, and oversensing.
Causes of Loss of Continuous Biventricular Pacing and Mean Time Elapsed After Implantation
| Cause of loss | Total | Time elapsed since implantation, years |
| Atrial arrhythmias | 13 (21.3) | 1.79±1.77 |
| LV lead dislodgement | 11 (18.0) | 1.14±1.33 |
| Failure of LV capture | 8 (13.1) | 2.97±1.68 |
| Recurrent ventricular arrhythmias | 5 (8.1) | 1.70±2.39 |
| Intrinsic conduction and oversensing | 5 (8.1) | 0.76±0.74 |
| Infection | 5 (8.1) | 3.82±3.04 |
| Extracardiac stimulation | 4 (6.5) | 0.76±0.88 |
| Loss of atrial sensing | 4 (6.5) | 1.39±1.57 |
| Failure of RV capture | 3 (4.9) | 3.89±1.96 |
| RV lead dislodgement | 2 (3.2) | 0.11±0.09 |
| Clinical intolerance | 1 (1.6) | 2.36 |
LV, left ventricular; RV, right ventricular.
Data are expressed as mean±standard deviation or no. (%).
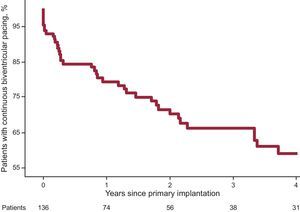
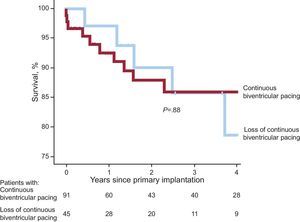
Figure 1 shows that most of the events involving loss of continuous biventricular pacing took place during the first year of follow-up; the incidence of pacing loss was 22% at 1 year after implantation, rising to 40% after 4 years of follow-up. There were no statistically significant differences in mortality between the groups with and without loss of continuous biventricular pacing (P=.88) (Fig. 2).
None of the baseline variables proved to be statistically significant predictors of loss of continuous biventricular pacing during follow-up.
Measures TakenDepending on the identified cause of loss of continuous biventricular pacing, the measures adopted in the attempt to restore it were as follows:
- •
Atrial tachyarrhythmias: this was the cause in 13 occasions. The most prevalent arrhythmia was atrial fibrillation with rapid ventricular response. The main measures adopted to restore continuous biventricular pacing were pharmacological cardioversion (generally with amiodarone) or electrical cardioversion in patients diagnosed with paroxysmal or persistent atrial fibrillation, and optimization of beta-blocker therapy in patients with permanent atrial fibrillation. Restoration of biventricular pacing of over 90% of the beats was not achieved in 3 patients. All 3 of these patients had chronic atrial fibrillation with repeat episodes of nonsymptomatic rapid ventricular response. Given that these patients were in NYHA functional class II and had biventricular pacing of 85% to 89%, they did not undergo atrioventricular node ablation to control heart rate and thus restore continuous biventricular pacing.
- •
Dislodgement of the LV lead: this was observed in 11 patients. All of the patients underwent surgical revision of the system with replacement or repositioning of the LV lead that had previously been implanted percutaneously. This was achieved in 9 patients. Satisfactory percutaneous reimplantation of a lead was not feasible in 2; of these, surgical implantation of the LV lead by means of thoracotomy was performed in 1 patient, while a history of previous coronary revascularization surgery in the circumflex artery territory made surgical epicardial lead placement inadvisable in the other.
- •
Failure of LV lead capture: this was the cause in 8 patientscases. In all of them, the pacing output of the coronary sinus lead was adjusted or the pacing vector was modified. In 3 patients, this measure did not allow for a safety margin between the capture threshold and phrenic nerve stimulation, and surgical revision was necessary, with repositioning of the LV lead at another site in the venous system. Continuous biventricular pacing could not be restored in only 1 patient; in this patient, we were unable to achieve an adequate capture threshold without phrenic nerve stimulation, despite surgical revision, and the patient refused to undergo an attempt at surgical implantation involving thoracotomy.
- •
Recurrent ventricular arrhythmias: the major cause of pacing loss was sensing of high-density premature ventricular contractions. This problem was solved by the introduction of antiarrhythmic drug therapy and the application of algorithms corresponding to the device in 4 patients. In 1 patient, continuous biventricular pacing could not be restored despite therapy and reprogramming of the device.
- •
Intrinsic conduction and oversensing: these problems occurred in 5 patients. The major cause of the loss was intrinsic atrioventricular conduction, which was corrected by reprogramming the device, specifically by shortening the atrioventricular interval and increasing the maximum and minimum follow-up rates. Oversensing of the T wave of the previous paced beat, which occurred in 1 patient, was corrected by reprogramming the sensitivity of the right ventricular lead.
- •
Infection: in the 5 cases of infection, the entire system was explanted and a cycle of specific antibiotic therapy was administered. Once the treatment had been completed and resolution of the infection had been confirmed, a new pacing system was implanted; in 1 of these patients, thoracotomy was performed for lead implantation.
- •
Extracardiac stimulation: 4 patients experienced highly symptomatic phrenic nerve stimulation, requiring temporary suspension of pacing with the LV lead. In each patient, the device was reprogrammed with modification of the pacing configuration. In 2 patients, checking the system and repositioning the LV lead within the coronary venous system were required, as there was no margin for programming between the capture and phrenic nerve stimulation thresholds.
- •
Loss of atrial sensing: this was observed in 4 patients. Continuous biventricular pacing was restored by replacing the right atrial lead due to dislodgement in 2 patients and by reprogramming atrial sensing in the remaining two.
- •
Failure of right ventricular capture: this cause was identified in 3 patients. In 2, the lead needed to be replaced due to suspected fracture and perforation, respectively; in the remaining patient, the increase in the pacing output of the lead was sufficient to achieve adequate therapy.
- •
Dislodgement of the right ventricular lead: in the 2 occurrences of dislodgement, surgical revision of the system was carried out and the problem lead was replaced.
- •
Intolerance to CRT: this occurred in only 1 patient. The basic symptoms were dyspnea and marked asthenia at CRT initiation. Clinical improvement was not achieved despite careful optimization of the parameters, and pacing with the LV lead had to be suspended.
In this contemporary series of consecutive patients who had undergone implantation of a CRT device: a) loss of continuous biventricular pacing during follow-up occurred in one third of the patients; b) in one half of these patients, the causes of the loss of continuous biventricular pacing were atrial arrhythmias, especially atrial fibrillation with rapid ventricular response, and problems with the LV lead, and c) continuous biventricular pacing could usually be restored by means of individualized interventions in each patient depending on the cause identified.
Importance of Continuous Biventricular PacingThe benefits of CRT are directly related to the maintenance of continuous biventricular pacing. Thus, rates of biventricular pacing close to 100% are associated with a significant reduction in mortality.15,16 Hayes et al.16 analyzed a cohort of 36 935 patients followed for a mean period of nearly 600 days from a remote device monitoring platform (LATITUDE, Boston Scientific). These authors observed a 24% reduction in mortality In patients with biventricular pacing greater than 99.6% with respect to the remaining patients. In contrast, in the quartile of patients with biventricular pacing lower than 95%, they detected an 19% increase in the mortality rate compared with the remaining quartiles. In the present study, analysis of mortality revealed no statistically significant differences between the groups, which could be related to the small sample size and to the definition of loss of continuous biventricular pacing as pacing less than 90%. Moreover, the benefits of continuous biventricular pacing do not affect overall mortality over the long term alone; the reduction of these benefits over the short term is also associated with clinical worsening of HF.16
Major Events Impeding Continuous Biventricular PacingIn this study, the causes of the loss of continuous biventricular pacing varied widely (Table 2). However, atrial arrhythmias (especially atrial fibrillation) and problems related to the LV lead caused approximately half of the events. In a series of 443 patients with CRT, Knight et al.17 obtained similar data. In their study, over a significantly shorter mean follow-up period (2.5 years), CRT loss occurred in 36% of the patients and was permanent in 12.4%. The cause of CRT loss was atrial arrhythmias in 50.3% of the patients and problems with LV capture in 27.3%. The higher incidence of events related to atrial arrhythmias in comparison to our series (50.3% vs 21.3%) could be explained in part by the smaller proportion of patients treated with beta-blockers in the series of Knight et al. (49% vs 85.3%). A number of reports14–19 have demonstrated that inadequate management of episodes of atrial fibrillation and other tachyarrhythmias can have a substantial negative impact on the clinical benefit provided by CRT in patients with HF. In patients with CRT, Marijon et al.20 measured atrial tachyarrhythmias by analyzing the recordings of the electrograms obtained with the device, and found an incidence of 27% during the first year after implantation. In their series, the only variable independently associated with new episodes of atrial arrhythmias was a history of atrial tachyarrhythmias. Remote monitoring can play a relevant role in evaluating the frequency of these arrhythmias and their negative impact on the percentage of biventricular pacing.16–19 In patients refractory to drug therapy, atrioventricular node ablation is an option to be considered.21
In our study, taken together, problems related to the LV lead, whether dislodgement or failure to capture, were the major cause of loss of continuous biventricular pacing (31.1% of the events). In this series, 14 patients (10%) required surgical revision to adequately restore LV capture, an incidence that is slightly higher than that documented in the study by Knight et al.17 (8%). The mean time lapse between implantation and identification of loss of continuous biventricular pacing due to LV lead dislodgement was relatively short in our series (1.14 [1.33] years). Landolina et al.22 analyzed the long-term complications and rate of surgical revisions in CRT in a cohort of 3253 patients. In that study, the rate of surgical revisions related to LV lead dislodgement was 2.3 events per 100 patient-years, and, on Kaplan-Meier analysis, surgical revisions were found to be more frequent in the 6 months after primary implantation. In contrast, in our series, extracardiac stimulation, most frequently phrenic nerve stimulation by the LV lead, was the cause in 6.5% of the events, half of which were corrected by reprogramming the pacing vector, and the other half (3.25% of the events) required surgical revision of the system. The utilization of LV leads based on novel designs, such as quadripolar leads, could significantly reduce the incidence of loss of continuous biventricular pacing related to failure to capture, lead dislodgement, and phrenic nerve stimulation.23
Despite the improvements in implantation techniques, cardiac device infections continue to be a relevant and serious problem. In our series, infections were the cause of loss of continuous biventricular pacing in 8.1% of the patients (5 events), an incidence somewhat higher than that reported by Knight et al.17 (3.1%).
Our study, like previous reports,17,22 shows that CRT device implantation is associated with a high rate of a variety of device-related events, and that most of these occur during the first months after implantation.
LimitationsThe major limitation of this study is due to its observational rather than randomized design, as well as to the fact that it involves a single center and a relatively small number of patients. However, in terms of the study's objectives, the data presented are highly significant with regard to the elevated incidence of problems that impede continuous biventricular pacing and the fact that, in most cases, they can be corrected. Our definition of loss of continuous biventricular pacing as the documentation between visits of biventricular pacing of less than 90% of the beats, when several authors have proposed that the goal should be pacing of over 95% of the beats,15,16 could have resulted in underestimation of the true incidence of the problem being evaluated. Moreover, with the routine use of remote monitoring systems for device follow-up (an approach that was not employed in this study), the incidence of patients with loss of continuous biventricular pacing could be expected to be even higher, since these systems permit closer monitoring of the percentage of biventricular pacing.
CONCLUSIONSThe documentation of suboptimal percentages of biventricular pacing in patients with CRT is a highly common and important problem with a very high incidence, especially during the first months after implantation. Close patient follow-up and adequate evaluation and management of the potential causes (especially of atrial arrhythmias and LV lead-related problems) usually enable optimal restoration of therapy, without compromising survival.
CONFLICTS OF INTERESTNone declared.