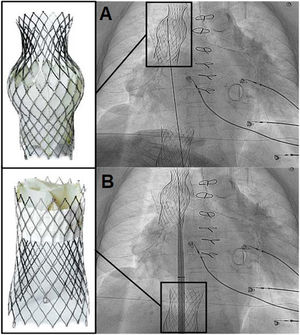
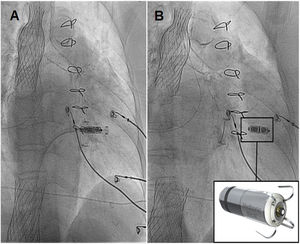
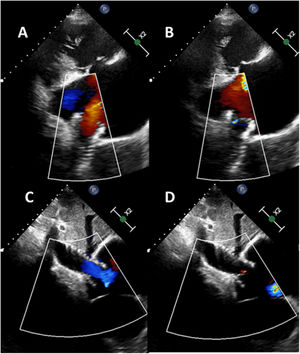
Tricuspid regurgitation (TR) has high morbidity and mortality. New percutaneous treatments have been designed for this disease, such as transcatheter bicaval valve implantation (CAVI), with promising results. However, cardiac pacing in this context represents a new challenge. We present the case of a 78-year-old woman with a mechanical mitral valve, with massive TR in overt heart failure and high surgical risk. After consenting to the procedure and its publication, the patient underwent CAVI with a TricValve (Products & Features, Austria): a system of 2 self-expanding biological valves that are implanted into both venae cavae. After the procedure, she remained in heart failure, attributed to atrial fibrillation with slow ventricular response. We decided to use a Micra-TPS-VR leadless pacemaker (LPM) (Medtronic, USA) to avoid the lead interfering with closure of the superior valve. The pacemaker was implanted in a way that avoided crossing the superior valve with the sheath and the dilator (figure 1) to prevent a potential tear of the prosthetic tissue in the opposite direction of the valve opening. Next, the system was advanced to the right ventricle for its release in a medioseptal position (figure 2), with excellent electrical parameters. Subsequent echocardiogram showed that both TricValve prostheses were working well after the procedure, with laminar flow in diastole and minimal regurgitation in systole (figure 2, superior valve above and inferior valve below). The patient's heart failure resolved, allowing her to be discharged from hospital. LPM implantation is feasible after CAVI and avoids the complications associated with the passage of pacing leads through the prosthetic valve of the superior vena cava. figure 3
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSAll authors participated in the writing, review, and approval of the manuscript.
CONFLICTS OF INTERESTA. Fontenla declares having received consultancy fees from Medtronic. The other authors declare no conflicts of interest.