Stent thrombosis (ST) is a life-threatening complication after stent implantation. Intravascular ultrasound is able to discern most causes of ST. The aim of this study was to compare intravascular ultrasound findings between bare-metal stents (BMS) and drug-eluting stents (DES) in patients with late (31 days to 1 year) or very late ST (> 1 year).
MethodsOf 250 consecutive patients with late or very late ST in 7 Spanish institutions, 114 patients (45.5% BMS and 54.5% DES) were imaged with intravascular ultrasound. Off-line intravascular ultrasound analysis was performed to assess malapposition, underexpansion, and neoatherosclerosis.
ResultsThe median time from stent implantation to ST was 4.0 years with BMS and 3.4 years with DES (P = .04). Isolated malapposition was similarly observed in both groups (36.5% vs 46.8%; P = .18) but was numerically lower with BMS (26.6% vs 48.0%; P = .07) in patients with very late ST. Isolated underexpansion was similarly observed in both groups (13.5% vs 11.3%; P = .47). Isolated neoatherosclerosis occurred only in patients with very late ST and was more prevalent with BMS (22.9%) than with DES (6.0%); P = .02. At 2.9 years’ follow-up, there were 0% and 6.9% cardiac deaths, respectively (P = .06) and recurrent ST occurred in 4.0% and 5.2% of patients, respectively (P = .60).
ConclusionsMalapposition was the most common finding in patients with late and very late ST and is more prevalent with DES in very late ST. In contrast, neoatherosclerosis was exclusively observed in patients with very late ST and mainly with BMS.
Keywords
Stent thrombosis (ST) is a rare but life-threatening complication that usually results in ST-segment elevation myocardial infarction. The mortality rate is around 20% to 40%.1,2 Definite late and very late ST are defined as the presence of angiographic or pathologic intrastent thrombus occurring later than 1 month after the index percutaneous coronary intervention (PCI).3 The incidence of ST has been reduced in the last few years by the emergence of new-generation drug-eluting stents (DES) and contemporary antithrombotic therapies.4–6
The etiology of ST is usually multifactorial.7 Intravascular ultrasound (IVUS) is an intracoronary imaging tool able to characterize vessel wall remodeling and to discern most causes of ST, such as persistent or late incomplete stent/strut apposition (malapposition), underexpansion, and neoatherosclerosis. Intravascular ultrasound can also predict cardiovascular events at follow-up in patients treated with IVUS-guided PCI.8 Current myocardial revascularization guidelines recommend the use of intravascular imaging techniques to detect stent-related mechanical problems (class IIa, level of evidence C).9 Assessment of causes of ST may help to select the best treatment strategy for each case. Treatment with balloon angioplasty without additional stent implantation has been associated with greater resolution of malapposition and stent underexpansion than treatment with additional stent implantation in the assessment of the posttreatment results with IVUS. In contrast, patients with neoatherosclerosis could benefit from additional stent implantation.10
Little is known about differences in the prevalence, timing, and causes of late and very late ST between bare-metal stent (BMS) and DES. The aim of this study was to compare the clinical, angiographic, and IVUS findings between BMS or DES in patients with definite late and very late ST.
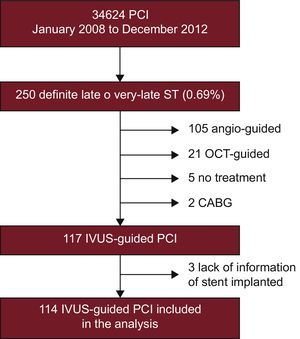
METHODSPopulation and Procedure CharacteristicsAll patients with angiographic late or very late ST (≥ 1 month) were prospectively included in 7 Spanish Institutions from January 2008 to December 2012. Late ST are those occurring between 31 days to 1 year after stent implantation, whereas very late ST are those occurring > 1 year after stent implantation.3 All Institutions participating in the study were high-volume centers (> 500 PCI/y) with high use of IVUS for complex PCI.11 A total of 250 consecutive eligible patients presented with definite late or very late ST as defined by the Academic Research Consortium (0.69% of all PCIs performed by all institutions participating in the study). Of these, 117 lesions in 116 patients were imaged with IVUS. Three of these patients were excluded from the analysis due to lack of information on the stent type. The patient flowchart is shown in Figure 1. Most of the excluded patients were those seeking medical attention during off-office hours or were hemodynamically unstable. This study was approved by the local ethics committee of all participating institutions and was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients.
Percutaneous coronary intervention was performed according to the standard practices in each participating center and the treatment of the ST was left to the operator's discretion after “on-line” evaluation of IVUS images.
Intravascular Ultrasound Acquisition and AnalysisIVUS imaging was performed after the restoration of Thrombolysis in Myocardial Infarction (TIMI) flow ≥ 2 with thrombus aspiration or percutaneous transluminal coronary balloon angioplasty. IVUS acquisition was performed with the Atlantis 40MHz catheter (Boston Scientific, Marlborough, MA, United States).
Image acquisition was done using an automated pullback system transducer with a pullback speed of 0.5mm/s except for 1 institution that performed IVUS recording at 1mm/s. The image data were digitally recorded for “off-line” analysis.
“Off-line” IVUS analysis was performed by 2 experienced analysts blinded to the type of stent implanted using quantitative IVUS analysis software (QIvus 3.0, Medis, Leiden, The Netherlands). All analyses were performed by a local core laboratory (BARCICORE-lab, Barcelona, Spain). The stent segment was defined by the stent edges. The proximal and distal reference segments were defined as the 5mm proximal and distal to the stent edges whenever possible.
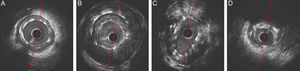
Before the quantitative analysis, the 2 operators were requested to qualitatively evaluate the IVUS pullback and to identify 5 IVUS findings: malapposition, aneurysms, stent fracture, stent underexpansion, and neoatherosclerosis. Malapposition was defined as a separation of at least 1 metallic strut from the vessel wall in the absence of a side branch. Aneurysms were defined as lesions that included all layers of the vessel wall with an external elastic membrane and lumen area > 50% larger than the proximal reference segment.12,13 Stent fracture was defined as a 0.5-mm gap within the stent segment. Stent underexpansion was defined when the minimal stent area was ≤ 80% of the reference lumen area. Neoatherosclerosis was defined as the presence of clear intrastent plaque with lipid or calcium echogenic characteristics.14,15 Examples of each IVUS finding are shown in Figure 2.
Quantitative measurements of lumen, stent, vessel (external elastic membrane), malapposition, and neointimal areas were performed according to standard procedures.12,16 The reference lumen area was obtained by the mean of the largest lumen area measured within the 5mm proximal and distal segments.12
Clinical Follow-upMajor adverse cardiac events at follow-up were defined as death, recurrent ST, or target lesion revascularization. Clinical events and follow-up information were obtained from telephone interviews with the patients or their relatives and from hospital records.
Statistical AnalysisStatistical analysis was performed using the SPSS Statistics package, version 20.0 (IBM Corp Armonk, New York, United States). The Kolmogorov-Smirnov test was used to evaluate the normality assumptions of all continuous variables. Continuous variables are reported as the mean ± 1 standard deviation or interquartile range when nonnormally distributed. Categorical variables are expressed as numbers and percentages. If the data were normally distributed, the Student t test was used to assess differences in continuous variables between the DES- and BMS-treated groups. The Wilcoxon test was used in nonnormally distributed variables. Comparisons between categorical variables were performed with the chi-square test. Event-free survival curves were generated with Kaplan-Meier analysis, and survival curves among groups were compared using the log-rank test. A 2-sided P value < .05 was considered significant.
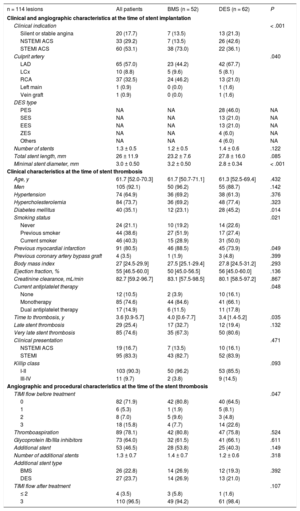
RESULTSBaseline Clinical and Angiographic Characteristics at the Time of Stent ImplantationA total of 114 lesions in 113 patients with late or very late ST were included in the present study: 52 (45.5%) were BMS and 62 (54.5%) were DES. Baseline clinical, angiographic and procedural characteristics are shown in Table 1. Bare-metal stents were more often implanted in patients with ST-segment elevation acute myocardial infarction (73.1% vs 36.1%; P < .01) and in the right coronary artery (46.2% vs 21.0%; P = .04) at the index procedure. The minimum stent diameter was higher in the BMS group than in the DES group (3.2 ± 0.5mm vs 2.8 ± 0.4mm; P < .01). First-generation DES were used in 67% of the patients in the DES group.
Clinical, Angiographic and Procedural Characteristics
| n = 114 lesions | All patients | BMS (n = 52) | DES (n = 62) | P |
|---|---|---|---|---|
| Clinical and angiographic characteristics at the time of stent implantation | ||||
| Clinical indication | < .001 | |||
| Silent or stable angina | 20 (17.7) | 7 (13.5) | 13 (21.3) | |
| NSTEMI ACS | 33 (29.2) | 7 (13.5) | 26 (42.6) | |
| STEMI ACS | 60 (53.1) | 38 (73.0) | 22 (36.1) | |
| Culprit artery | .040 | |||
| LAD | 65 (57.0) | 23 (44.2) | 42 (67.7) | |
| LCx | 10 (8.8) | 5 (9.6) | 5 (8.1) | |
| RCA | 37 (32.5) | 24 (46.2) | 13 (21.0) | |
| Left main | 1 (0.9) | 0 (0.0) | 1 (1.6) | |
| Vein graft | 1 (0.9) | 0 (0.0) | 1 (1.6) | |
| DES type | ||||
| PES | NA | NA | 28 (46.0) | NA |
| SES | NA | NA | 13 (21.0) | NA |
| EES | NA | NA | 13 (21.0) | NA |
| ZES | NA | NA | 4 (6.0) | NA |
| Others | NA | NA | 4 (6.0) | NA |
| Number of stents | 1.3 ± 0.5 | 1.2 ± 0.5 | 1.4 ± 0.6 | .122 |
| Total stent length, mm | 26 ± 11.9 | 23.2 ± 7.6 | 27.8 ± 16.0 | .085 |
| Minimal stent diameter, mm | 3.0 ± 0.50 | 3.2 ± 0.50 | 2.8 ± 0.34 | < .001 |
| Clinical characteristics at the time of stent thrombosis | ||||
| Age, y | 61.7 [52.0-70.3] | 61.7 [50.7-71.1] | 61.3 [52.5-69.4] | .432 |
| Men | 105 (92.1) | 50 (96.2) | 55 (88.7) | .142 |
| Hypertension | 74 (64.9) | 36 (69.2) | 38 (61.3) | .376 |
| Hypercholesterolemia | 84 (73.7) | 36 (69.2) | 48 (77.4) | .323 |
| Diabetes mellitus | 40 (35.1) | 12 (23.1) | 28 (45.2) | .014 |
| Smoking status | .021 | |||
| Never | 24 (21.1) | 10 (19.2) | 14 (22.6) | |
| Previous smoker | 44 (38.6) | 27 (51.9) | 17 (27.4) | |
| Current smoker | 46 (40.3) | 15 (28.9) | 31 (50.0) | |
| Previous myocardial infarction | 91 (80.5) | 46 (88.5) | 45 (73.9) | .049 |
| Previous coronary artery bypass graft | 4 (3.5) | 1 (1.9) | 3 (4.8) | .399 |
| Body mass index | 27 [24.5-29.9] | 27.5 [25.1-29.4] | 27.8 [24.5-31.2] | .293 |
| Ejection fraction, % | 55 [46.5-60.0] | 50 [45.0-56.5] | 56 [45.0-60.0] | .136 |
| Creatinine clearance, mL/min | 82.7 [59.2-96.7] | 83.1 [57.5-98.5] | 80.1 [58.5-97.2] | .867 |
| Current antiplatelet therapy | .048 | |||
| None | 12 (10.5) | 2 (3.9) | 10 (16.1) | |
| Monotherapy | 85 (74.6) | 44 (84.6) | 41 (66.1) | |
| Dual antiplatelet therapy | 17 (14.9) | 6 (11.5) | 11 (17.8) | |
| Time to thrombosis, y | 3.6 [0.9-5.7] | 4.0 [0.6-7.7] | 3.4 [1.4-5.2] | .035 |
| Late stent thrombosis | 29 (25.4) | 17 (32.7) | 12 (19.4) | .132 |
| Very late stent thrombosis | 85 (74.6) | 35 (67.3) | 50 (80.6) | |
| Clinical presentation | .471 | |||
| NSTEMI ACS | 19 (16.7) | 7 (13.5) | 10 (16.1) | |
| STEMI | 95 (83.3) | 43 (82.7) | 52 (83.9) | |
| Killip class | .093 | |||
| I-II | 103 (90.3) | 50 (96.2) | 53 (85.5) | |
| III-IV | 11 (9.7) | 2 (3.8) | 9 (14.5) | |
| Angiographic and procedural characteristics at the time of the stent thrombosis | ||||
| TIMI flow before treatment | .047 | |||
| 0 | 82 (71.9) | 42 (80.8) | 40 (64.5) | |
| 1 | 6 (5.3) | 1 (1.9) | 5 (8.1) | |
| 2 | 8 (7.0) | 5 (9.6) | 3 (4.8) | |
| 3 | 18 (15.8) | 4 (7.7) | 14 (22.6) | |
| Thromboaspiration | 89 (78.1) | 42 (80.8) | 47 (75.8) | .524 |
| Glycoprotein IIb/IIIa inhibitors | 73 (64.0) | 32 (61.5) | 41 (66.1) | .611 |
| Additional stent | 53 (46.5) | 28 (53.8) | 25 (40.3) | .149 |
| Number of additional stents | 1.3 ± 0.7 | 1.4 ± 0.7 | 1.2 ± 0.6 | .318 |
| Additional stent type | ||||
| BMS | 26 (22.8) | 14 (26.9) | 12 (19.3) | .392 |
| DES | 27 (23.7) | 14 (26.9) | 13 (21.0) | |
| TIMI flow after treatment | .107 | |||
| ≤ 2 | 4 (3.5) | 3 (5.8) | 1 (1.6) | |
| 3 | 110 (96.5) | 49 (94.2) | 61 (98.4) | |
ACS, acute coronary syndrome; BMS, bare-metal stents; DES, drug-eluting stents; EES, everolimus-eluting stent; LAD, left anterior descending artery; LCx, left circumflex; NA, non applicable; NSTEMI, non—ST-segment elevation myocardial infarction; PES, paclitaxel-eluting stent; RCA, right coronary artery; SES, sirolimus-eluting stent; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction; ZES, zotarolimus-eluting stent.
Data are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
Overall, ST occurred at a median of 3.6 years (range, 0.9-5.7 years) after the index PCI. However, ST occurred later in the BMS group (4.0 years; range, 0.6-7.7 years) than in the DES group (3.4 years; range, 1.4-5.2 years); P = .04. The clinical presentation at the time of ST was acute ST-segment elevation myocardial infarction in 83.3% of patients and non—ST-segment elevation myocardial infarction in 16.7% of patients, with no differences between treatment groups.
Clinical characteristics at the time of the ST were similar between the 2 stent types except for diabetes mellitus (23.1% in the BMS group vs 45.2% in the DES group, P = .01), smoking status (28.9% of current smokers in the BMS group vs 50.0% in the DES group; P = .02), and history of previous myocardial infarction (88.5% vs 73.9%: P = .05), respectively. Most of the patients were on single antiplatelet therapy at admission (74.6%). A total of 10.5% were not receiving any antiplatelet drug; among these, there was a larger proportion of patients in the BMS group (16.1%) than in the DES group (3.9%); P = .05.
At the time of the ST, BMS patients more often had total occlusion of the target vessel with TIMI flow 0 before treatment (80.8% vs 64.5%; P = .05, respectively). A total of 53 lesions (46.5%) were treated with an additional stent implantation, with a numeric difference between groups (53.8% vs 40.3%; P = .15, respectively).
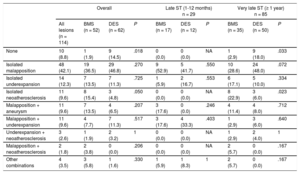
Intravascular Ultrasound FindingsQualitative IVUS findings including all possible combinations of the selected IVUS parameters are summarized in Table 2. All patients had IVUS acquisition before treatment. In 10 of the patients (8.6%), none of the predefined qualitative IVUS findings were observed: 1.9% in the BMS group vs 14.5% in the DES group, P = .02.
Qualitative Intravascular Ultrasound Findings
| Overall | Late ST (1-12 months) n = 29 | Very late ST (≥ 1 year) n = 85 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All lesions (n = 114) | BMS (n = 52) | DES (n = 62) | P | BMS (n = 17) | DES (n = 12) | P | BMS (n = 35) | DES (n = 50) | P | |
| None | 10 (8.8) | 1 (1.9) | 9 (14.5) | .018 | 0 (0.0) | 0 (0.0) | NA | 1 (2.9) | 9 (18.0) | .033 |
| Isolated malapposition | 48 (42.1) | 19 (36.5) | 29 (46.8) | .270 | 9 (52.9) | 5 (41.7) | .550 | 10 (28.6) | 24 (48.0) | .072 |
| Isolated underexpansion | 14 (12.3) | 7 (13.5) | 7 (11.3) | .725 | 1 (5.9) | 2 (16.7) | .553 | 6 (17.1) | 5 (10.0) | .334 |
| Isolated neoatherosclerosis | 11 (9.6) | 8 (15.4) | 3 (4.8) | .050 | 0 (0.0) | 0 (0.0) | NA | 8 (22.9) | 3 (6.0) | .023 |
| Malapposition + aneurysm | 11 (9.6) | 7 (13.5) | 4 (6.5) | .207 | 3 (17.6) | 0 (0.0) | .246 | 4 (11.4) | 4 (8.0) | .712 |
| Malapposition + underexpansion | 11 (9.6) | 4 (7.7) | 7 (11.3) | .517 | 3 (17.6) | 4 (33.3) | .403 | 1 (2.9) | 3 (6.0) | .640 |
| Underexpansion + neoatherosclerosis | 3 (2.6) | 1 (1.9) | 2 (3.2) | 1 | 0 (0.0) | 0 (0.0) | NA | 1 (2.9) | 2 (4.0) | 1 |
| Malapposition + neoatherosclerosis | 2 (1.8) | 2 (3.8) | 0 (0.0) | .206 | 0 (0.0) | 0 (0.0) | NA | 2 (5.7) | 0 (0.0) | .167 |
| Other combinations | 4 (3.5) | 3 (5.8) | 1 (1.6) | .330 | 1 (5.9) | 1 (8.3) | 1 | 2 (5.7) | 0 (0.0) | .167 |
BMS, bare-metal stents; DES, drug-eluting stents; NA, non applicable; ST, stent thrombosis.
Data are expressed as No. (%).
Isolated malapposition was found in 48 (42.1%) patients, isolated underexpansion in 14 (12.3%) and isolated neoatherosclerosis in 11 (9.6%). There was only 1 stent fracture (0.9%), in a patient in the DES group. Isolated malapposition was numerically lower in the BMS group than in the DES group (36.5% vs 46.8%; P = .18). When only patients with very late ST were analyzed, isolated malapposition was 20% lower in BMS-treated patients (28.6% vs 48.0%; P = .07, respectively). The combination of malapposition and aneurysm tended to be higher in the BMS group (13.5% vs 6.5%; P = .17). Isolated neoatherosclerosis was exclusively observed in patients with very late ST and was more frequent in the BMS group (15.4% vs 4.8%: P = .05).
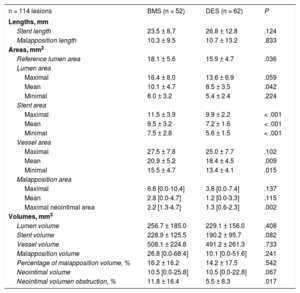
Quantitative IVUS data are summarized in Table 3. Differences were observed regarding the mean lumen area (10.1 ± 4.7mm2 vs 8.5 ± 3.5mm2; P = .04), mean stent area (9.5 ± 3.2mm2 vs 7.2 ± 1.6mm2, P < .01), and mean vessel area (20.9 ± 5.2mm2 vs 18.4 ± 4.5mm2; P = .01), with areas being significantly larger in the BMS than in the DES group, respectively. The malapposition length was similar between BMS (10.3 ± 9.5) and DES (10.7 ± 13.2); P = .83. However, the maximal malapposition area was numerically larger in the BMS group (6.6mm2; range 0-10.4mm2) than in the DES group (3.8mm2; range 0-7.4); P = .14.
Quantitative Intravascular Ultrasound Findings
| n = 114 lesions | BMS (n = 52) | DES (n = 62) | P |
|---|---|---|---|
| Lengths, mm | |||
| Stent length | 23.5 ± 8.7 | 26.8 ± 12.8 | .124 |
| Malapposition length | 10.3 ± 9.5 | 10.7 ± 13.2 | .833 |
| Areas, mm2 | |||
| Reference lumen area | 18.1 ± 5.6 | 15.9 ± 4.7 | .036 |
| Lumen area | |||
| Maximal | 16.4 ± 8.0 | 13.6 ± 6.9 | .059 |
| Mean | 10.1 ± 4.7 | 8.5 ± 3.5 | .042 |
| Minimal | 6.0 ± 3.2 | 5.4 ± 2.4 | .224 |
| Stent area | |||
| Maximal | 11.5 ± 3.9 | 9.9 ± 2.2 | < .001 |
| Mean | 9.5 ± 3.2 | 7.2 ± 1.6 | < .001 |
| Minimal | 7.5 ± 2.8 | 5.6 ± 1.5 | < .001 |
| Vessel area | |||
| Maximal | 27.5 ± 7.8 | 25.0 ± 7.7 | .102 |
| Mean | 20.9 ± 5.2 | 18.4 ± 4.5 | .009 |
| Minimal | 15.5 ± 4.7 | 13.4 ± 4.1 | .015 |
| Malapposition area | |||
| Maximal | 6.6 [0.0-10.4] | 3.8 [0.0-7.4] | .137 |
| Mean | 2.8 [0.0-4.7] | 1.2 [0.0-3.3] | .115 |
| Maximal neointimal area | 2.2 [1.3-4.7] | 1.3 [0.6-2.3] | .002 |
| Volumes, mm3 | |||
| Lumen volume | 256.7 ± 185.0 | 229.1 ± 156.0 | .408 |
| Stent volume | 228.9 ± 125.5 | 190.2 ± 95.7 | .082 |
| Vessel volume | 508.1 ± 224.8 | 491.2 ± 261.3 | .733 |
| Malapposition volume | 26.8 [0.0-68.4] | 10.1 [0.0-51.6] | .241 |
| Percentage of malapposition volume, % | 16.2 ± 16.2 | 14.2 ± 17.5 | .542 |
| Neointimal volume | 10.5 [0.0-25.8] | 10.5 [0.0-22.8] | .067 |
| Neointimal volumen obstruction, % | 11.8 ± 16.4 | 5.5 ± 8.3 | .017 |
BMS, bare-metal stents; DES, drug-eluting stents.
Unless otherwise indicated, data are expressed as mean ± standard deviation or median [interquartile range].
The maximal neointimal area was also larger in the BMS group (2.2mm2; range, 1.3-4.7mm2) than in the DES group (1.3mm2, range 0.6-2.3mm2); P < .01. Neointimal volume obstruction, defined as the neointimal volume divided by the stent volume, was also significantly larger in the BMS group (11.8% ± 16.4% vs 5.5% ± 8.3%; P = .02).
OutcomesClinical data were available in 107 patients (94.7%) with a median follow-up of 2.9 years (range, 1.9-4.9). During follow-up, there were 11 deaths (10.3%): 2 (4.0%) in the BMS group vs 9 (15.5%) in the DES group (P = .06). Only 4 deaths (3.7%) were from cardiac or unknown causes, 0 in the BMS group vs 4 (6.9%) in the DES group (P = .06). Target lesion revascularization was observed in a total of 6 patients (5.1%): 2 (4.0%) in the BMS group vs 4 (6.9%) in the DES group (P = .42). Recurrent definite or probable ST occurred in 5 patients (4.7%): 2 (4.0%) in the BMS group and 3 (5.2%) in the DES group (P = .60). All patients presenting with recurrent ST had malapposition at the time of the first ST. Treatment of the first ST had been balloon angioplasty without additional stent implantation in 1 patient and additional stent implantation in 4 patients. Immediately after PCI of the first ST, IVUS showed persistent malapposition in 4 of the patients (80.0%). Clinical presentation of the recurrent ST was ST-segment elevation myocardial infarction in 2 patients, non—ST-segment elevation myocardial infarction in 2 patients, and sudden cardiac death in 1 patient. Figure 3 shows the Kaplan-Meier survival curves for cardiac death, target lesion revascularization, and recurrent definite or probable ST, showing similar clinical outcomes between treatment groups regarding the prespecified major adverse cardiac events at follow-up.
DISCUSSIONThe main findings of the present study were: a) IVUS imaging in patients with late and very late ST identified a large number of those with mechanical causes of ST; b) malapposition was the most common finding in patients with late or very late ST, being observed in > 60% of patients and more frequently in the DES group; c) neoatherosclerosis occurred exclusively in patients with very late ST and was more frequent in the BMS group; and d) patients with late or very late ST treated with IVUS-guided strategies had favorable outcomes for both types of stent at mid-term follow-up.
To our knowledge, this is the largest study analyzing IVUS differences between BMS and DES in patients with late and very late ST. It is well known that the causes of ST are multifactorial and include patient-related factors, procedural factors, antiplatelet therapy, and device-specific factors. It has been reported that mechanical causes of early ST (< 1 month) usually consist of stent underexpansion, the presence of residual dissection, and impaired TIMI flow at the end of the procedure.17 In contrast, delayed vessel healing, positive vessel remodeling with late acquired malapposition, excessive neointimal response, and neoatherosclerosis have been associated with late and very late ST.18–21 IVUS-guided PCI is associated with better clinical outcomes at follow-up. A recent meta-analysis that included more than 25 000 patients demonstrated that IVUS-guided PCI was associated with a lower risk of death, myocardial infarction, target lesion revascularization, and ST after DES implantation.22
Malapposition is the most common finding observed in patients with ST.23–25 However, its importance is still controversial, especially regarding the extension that becomes clinically relevant and that can contribute to recurrent ST.25 It is known that the incidence of malapposition is higher in DES than in BMS in event-free patients as assessed by IVUS at 6 to 9 months.26 In that study, patients with malapposition had a higher risk of developing late or very late ST at follow-up.26 Kosonen et al.,27 found malapposition in 50% of the patients with very late ST as assessed by IVUS, being significantly more prevalent in patients treated with DES. In the same study, malapposition was more extensive in the DES group with larger maximal malapposition areas and longer malapposition length.27 In the present study, malapposition was also the most common finding in patients with ST in both the DES and BMS groups. The proportion of patients with malapposition was numerically larger in the DES group, especially in patients with very late ST. Nevertheless, there were no differences between groups regarding malapposition length, area, or volume.
Incomplete stent apposition can be persistent, related to inadequate stent implantation, or acquired due to positive vessel remodeling or thrombus dissolution after PCI. Guo et al.28 reported malapposition in 30% to 40% of patients immediately after primary PCI treated with DES or BMS. Of these, 40% of cases of malapposition resolved at 13 months. The incidence of late malapposition was higher in DES than in BMS, but no deaths or ST related to malapposition were reported in that study.
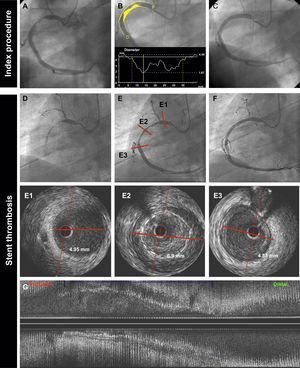
The presence of coronary aneurysms has also been associated with ST.29 In our study, the combination of malapposition and aneurysms was numerically higher in the BMS group (13.5% vs 6.5%) but this difference was not statistically significant. This finding can be explained by the preference of operators to implant BMS in large vessels not suitable for optimal stent apposition. An example of a patient with very late ST of a BMS implanted in a large right coronary artery is shown in Figure 4.
Very late BMS thrombosis caused by probable positive vessel remodeling. A 69-year-old man with non—ST-segment elevation myocardial infarction. The baseline angiography (A,B,C) showed a thrombotic lesion in the proximal right coronary artery (A). B: quantitative coronary angiography showed a reference vessel diameter of 4.84mm. P and D are the proximal and distal reference vessel diameters, respectively. C: the patient was treated with a 4.5 × 38mm BMS. D: at 18 months, the patient presented with very late ST. Angiography showed thrombotic occlusion of the artery. E: flow was restored after thrombus aspiration. IVUS imaging (E1, E2, E3, G) showed proximal and distal reference lumen diameters of 4.9mm (E1, E3); the stented segment showed probable positive vessel remodeling (lumen diameter 6.9mm) with large malapposition (E2, G: axial and longitudinal view respectively). The patient was treated with a 6.0 × 15mm noncompliant balloon. BMS, bare-metal stent; IVUS, intravascular ultrasound; ST, stent thrombosis.
The present study shows that neointimal proliferation and neoatherosclerosis were significantly more common in ST cases in the BMS group. Lee et al.30 described neointimal rupture within the stents in 100% of BMS-related very late ST and 43.5% of DES-related very late ST; Pesarini et al.31 observed plaque rupture in 78% vs 8% of patients with late or very late ST, respectively. Kang et al.32 observed neoatherosclerotic plaques in 70% of 33 very late ST in DES and BMS imaged with optical coherence tomography. This discrepancy between IVUS and optical coherence tomography findings can be explained by the low axial resolution of IVUS compared with optical coherence tomography, hampering characterization of the neointimal tissue in the stent and identification of the amount of plaque.32 Optical coherence tomography is the best imaging technique to assess strut coverage, malapposition, stent underexpansion, and neoatherosclerosis.33 However, in patients with ST, the presence of persistent thrombus may hamper the assessment of these findings and, in most cases, optical coherence tomography is unable to assess the external elastic membrane and the vessel wall reaction.
LimitationsFirst, this study is observational. All comparisons are only hypothesis-generating observations. Second, there may be an inclusion bias since IVUS was only performed in 50% of patients with ST presenting at in our institutions. Most of the excluded patients were admitted during off-office hours or were not imaged with IVUS due to hemodynamic instability. The exclusion of this high-risk group of patients could have influenced the study results. Moreover, first-generation DES were used in 67% of patients in the DES group. Therefore, further research is needed to extend these results to new-generation DES. Finally, IVUS was not performed at the index procedure; therefore, we were unable to distinguish between persistent or acquired malapposition.
CONCLUSIONSIVUS imaging in patients with late and very late ST was able to identify a large number of cases with mechanical causes of ST. Differences were found in the mechanical causes of ST between BMS and DES according to IVUS. Malapposition was the most common finding in patients with ST and was more often observed in patients with very late ST in DES. In contrast, neoatherosclerosis was exclusively observed in patients with very late ST and more frequently in patients with BMS. Patients with late and very late ST treated with IVUS-guided procedures had favorable clinical outcomes at mid-term follow-up. Therefore, knowledge of the causes could lead to better treatment of the ST.
- –
It is well known that the causes of ST are multifactorial. Intravascular ultrasound is an intracoronary imaging tool able to discern most of the causes of ST, such as malapposition, underexpansion, and neoatherosclerosis.
- –
Nevertheless, few studies have assessed differences in IVUS findings between BMS and DES in late or very late ST. Moreover, the incidence of the main IVUS findings assessed differ slightly between them.
- –
A previous study suggested that IVUS-guided treatment of ST was associated with better outcomes. Therefore, IVUS assessment of mechanical causes of ST may improve diagnosis and aid in selection of the best treatment strategy for each patient.
- –
This is the largest study to date analyzing IVUS differences between BMS and DES in patients with late and very late ST.
- –
This study shows that malapposition was the most common finding in all ST, contributing mostly to ST in DES, whereas neoatherosclerosis was mainly found in ST in BMS. These findings suggest that different mechanisms underlie ST development, depending on the stent type.
- –
Patients with late and very late ST treated with IVUS-guided strategies have favorable outcomes.
A. Sánchez-Recalde is Associate Editor of Revista Española de Cardiología.