Bioresorbable vascular scaffolds (BVS) have the potential to restore vasomotion but the clinical implications are unknown. We sought to evaluate angina and ischemia in the long-term in patients treated with BVS and metallic drug-eluting stents (mDES).
MethodsMulticenter study including patients with 24 ± 6 months of uneventful follow-up, in which stress echocardiography was performed and functional status was assessed by the Seattle Angina Questionnaire (SAQ). The primary endpoint was a positive result in stress echocardiography.
ResultsThe study included 102 patients treated with BVS and 106 with mDES. There were no differences in the patients’ baseline characteristics. Recurrent angina was found in 18 patients (17.6%) in the BVS group vs 25 (23.5%) in the mDES group (P = .37), but SAQ results were significantly better in the BVS group (angina frequency 96.0 ± 8.0 vs 89.2 ± 29.7; P = .02). Stress echocardiography was positive in 11/92 (11.9%) of BVS patients vs 9/96 (9.4%) of mDES patients in the (P = .71) and angina was induced in 2/102 (1.9%) vs 7/106 (6.6%) (P = .18), respectively, but exercise performance was better in the BVS group even in those with positive tests (exercise duration 9.0 ± 2.0minutes vs 7.7 ± 1.8minutes; P = .02). A propensity score matching analysis yielded similar results.
ConclusionsThe primary endpoint was similar in both groups. In addition, recurrent angina was similar in patients with BVS and mDES. The better functional status, assessed by means of SAQ and exercise performance, detected in patients receiving BVS should be confirmed in further studies.
Keywords
Recurrent angina after percutaneous revascularization with drug-eluting stents remains a clinically challenging problem.1,2 One of the causes is the abnormal vasomotion at the stented segment and potentially linked microvascular dysfunction.3 In this regard, bioresorbable vascular scaffolds (BVS) have the potential to overcome the inherent limitations of metallic drug-eluting stents (mDES).4–12 In the ABSORB II trial, a post hoc analysis showed that the site-reported cumulative rate of recurrent or worsening angina at 12 months was less common with the bioresorbable scaffold than with the cobalt–chromium stent.13 However, in that trial, no differences in the results of the Seattle Angina Questionnaire (SAQ) were found at 12 months or 3 years.13,14 In the larger ABSORB III trial, no differences in patient-reported angina rates were observed at 12 months with the metallic everolimus-eluting stent and BVS.15
In this multicenter study, we sought to evaluate recurrent angina, functional status, and ischemia in stress echocardiography (ECHO) beyond 1 year after implantation in a carefully selected population to ensure adequate interpretation of symptoms and tests results.
METHODSPatient PopulationA multicenter study was conducted in 11 tertiary hospitals, retrospective in the phase of patient selection for inclusion and prospective in the performance of all study procedures, including clinical evaluation and echocardiographic stress tests.
An initial screening was performed in all institutions in all patients undergoing percutaneous revascularization with BVS or mDES 24 ± 6 months previously, selecting those with an uneventful outcome (no death, acute coronary syndrome, stroke, or revascularization) up to the screening date. In a second stage, patients were matched by age and were then selected if they met the following criteria: a) treated with BVS only or with mDES only, not mixed; b) no primary angioplasty procedures; c) complete revascularization achieved (no lesions > 50% in vessels > 1.5mm left untreated); d) no bifurcations treated; e) vessels without distal diffuse disease according to visual assessment; f) no previous history of infarction, percutaneous coronary intervention, or coronary artery bypass graft; g) no left bundle branch block on electrocardiography (ECG); h) no severe left ventricular hypertrophy; i) no moderate or severe valvular heart disease; j) no treatment with digoxin; k) left ventricular ejection fraction > 50%; l) no akinesia in myocardial segments subtended by treated vessels; m) no intermittent claudication; n) no chronic pulmonary disease; o) no orthopedic limitation for exercise (ie, arthritis); p) no chronic analgesic treatment; q) no contraindications to exercise testing; r) no illnesses or conditions impeding appropriate participation in the study procedures. Signed written consent was required from all patients for study procedures.
The aims of this set of criteria were the following: a) to avoid the inclusion of patients with significant in-stent restenosis. Only patients with an uneventful follow-up for > 18 months were included, since most significant restenosis is detected and treated in the first 18 months. Moreover, beyond this time point, BVS scaffolds, although not fully absorbed, have supposedly lost most of their mechanical integrity; b) to avoid the inclusion of patients presenting all other potential sources of ischemia other than the stented segment, such as those related to untreated lesions either in the target vessel or in other vessels, distal disease in the target vessel, or jailed side branches; c) to prevent the inclusion of patients showing well-known causes of false-positive or false negative results in ECG or echocardiographic stress tests; d) to prevent the inclusion of patients with conditions that preclude the appropriate performance and interpretation of an exercise test either for ECG results, echocardiographic findings, or symptoms, and e) finally, because patients treated with BVS were significantly younger than those treated with metallic DES, an age-matching process was required.
The study was approved by each institutional review board.
Symptomatic EvaluationPatients were asked about the presence and characteristics of any kind of symptoms during follow-up after percutaneous coronary intervention. The SAQs were collected from all patients, with or without recurrent symptoms. The SAQ is scored by assigning each response an ordinal value, beginning with 1 for the response that implies the lowest level of functioning, and summing across items within each of the 5 scales.16 Scale scores are then transformed to a 0 to 100 range by subtracting the lowest possible scale score, dividing by the range of the scale, and multiplying by 100. Because each scale monitors a single dimension of coronary artery disease, no summary score is generated. The clinical cardiologists responsible for these activities were blinded to the treatment group.
Protocol for Exercise Test With Electrocardiographic RecordingExercise testing, including ECG recording, was performed according to the Bruce protocol treadmill test. Termination of the test was at the discretion of the attending staff but strictly followed guidelines and good practice recommendations.17 The following variables were recorded: a) time of exercise; b) percentage of maximum heart rate achieved; c) metabolic equivalents; d) peak double product. A test was labeled as clinically positive when symptoms compatible with angina were reported by the patient during or immediately after the exercise. A test was considered ECG-positive if ST depression of 0.1mV (1mm) or more, measured 80 msec from the J point, was detected during exercise or immediately after. The staff performing and interpreting these tests were unaware of the patient's treatment group.
Protocol for Stress EchocardiographyTwo-dimensional transthoracic ECHO from apical and paraesternal views was performed before exercise testing and immediately after (< 1minute).18 In every case, 5 ECHO views were collected (long and short axis from the parasternal view and 2, 3 and 4 chambers from the apical view). A digital set of images in a 4 per screen format were shown for comparative analysis between rest and peak stress recordings. Contractility was assessed according to the 16 myocardial segments model. A positive ECHO was considered when abnormalities in contractility appeared on exercise. Studies were collected at the coordination center for final interpretation. For the clinical evaluation, the echocardiographers were blinded to the patients’ group allocation.
Study EndpointsThe primary objective of the study was a positive result in stress ECHO (detection of abnormalities in contractility suggestive of ischemia) at 24 ± 6 months. The secondary objectives were SAQ results, the incidence of angina at any time during follow-up, the presence of angina in stress ECHO and exercise performance (duration and double product) at 24 ± 6 months.
Statistical AnalysisContinuous variables are presented as mean ± standard deviation or median [interquartile range]. Categorical variables are expressed as percentages. Continuous variables were compared with the Student t test if they followed a normal distribution and with Wilcoxon tests when they did not (assessment of the type of distribution by the Kolmogorov-Smirnov test). Categorical variables were compared with the chi-square test or the Fisher exact test, as required. An additional analysis was conducted by means of a propensity score matching, pairing patients treated with BVS and mDES.19 The propensity score matching process was conducted with all the variables listed in Table 1 and the center was entered as covariates for deriving the propensity scores. This procedure involved 3 stages: a) propensity scores were estimated using logistic regression in which BVS was used as the outcome variable and all the covariates as predictors; b) patients were matched using simple 1:1 nearest neighbor matching. To exclude bad matches, we imposed a caliper of 0.2 of the standard deviation of the logit of the propensity score; c) a series of model adequacy checks was performed to check whether balance on the covariates was achieved through the matching procedure. Standardized differences were calculated for all covariates before and after matching to assess balance after matching. A standardized difference < 10% for a given covariate indicates a relatively low imbalance. The “propensity score matching” custom dialogue was used in conjunction with SPSS version 19 (IBM, Armonk, New York, United States). The propensity score matching program performs all analyses in R (R Foundation for Statistical Computing, Vienna, Austria) through the SPSS R-Plugin (version 2.10.1). A P value < .05 was considered statistically significant. All statistical analyses were performed using SPSS version 19 for Windows.
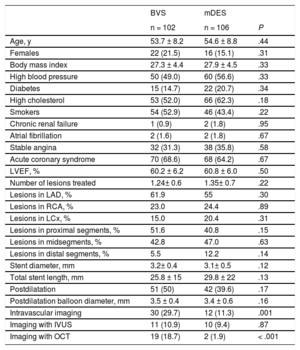
Baseline Clinical and Procedural Characteristics
| BVS | mDES | ||
|---|---|---|---|
| n = 102 | n = 106 | P | |
| Age, y | 53.7 ± 8.2 | 54.6 ± 8.8 | .44 |
| Females | 22 (21.5) | 16 (15.1) | .31 |
| Body mass index | 27.3 ± 4.4 | 27.9 ± 4.5 | .33 |
| High blood pressure | 50 (49.0) | 60 (56.6) | .33 |
| Diabetes | 15 (14.7) | 22 (20.7) | .34 |
| High cholesterol | 53 (52.0) | 66 (62.3) | .18 |
| Smokers | 54 (52.9) | 46 (43.4) | .22 |
| Chronic renal failure | 1 (0.9) | 2 (1.8) | .95 |
| Atrial fibrillation | 2 (1.6) | 2 (1.8) | .67 |
| Stable angina | 32 (31.3) | 38 (35.8) | .58 |
| Acute coronary syndrome | 70 (68.6) | 68 (64.2) | .67 |
| LVEF, % | 60.2 ± 6.2 | 60.8 ± 6.0 | .50 |
| Number of lesions treated | 1.24± 0.6 | 1.35± 0.7 | .22 |
| Lesions in LAD, % | 61.9 | 55 | .30 |
| Lesions in RCA, % | 23.0 | 24.4 | .89 |
| Lesions in LCx, % | 15.0 | 20.4 | .31 |
| Lesions in proximal segments, % | 51.6 | 40.8 | .15 |
| Lesions in midsegments, % | 42.8 | 47.0 | .63 |
| Lesions in distal segments, % | 5.5 | 12.2 | .14 |
| Stent diameter, mm | 3.2± 0.4 | 3.1± 0.5 | .12 |
| Total stent length, mm | 25.8 ± 15 | 29.8 ± 22 | .13 |
| Postdilatation | 51 (50) | 42 (39.6) | .17 |
| Postdilatation balloon diameter, mm | 3.5 ± 0.4 | 3.4 ± 0.6 | .16 |
| Intravascular imaging | 30 (29.7) | 12 (11.3) | .001 |
| Imaging with IVUS | 11 (10.9) | 10 (9.4) | .87 |
| Imaging with OCT | 19 (18.7) | 2 (1.9) | < .001 |
BVS, bioresorbable vascular scaffold; IVUS, intrasvascular ultrasound; LAD, left anterior descending artery; LCx, left circumflex artery; LVEF, left ventricular ejection fraction; mDES, metallic drug-eluting stent; OCT, optical coherence tomography; RCA; right coronary artery.
Unless otherwise indicated, values are expressed as no. (%) or mean ± standard deviation.
A total of 208 patients were included in the study. The baseline clinical and procedural characteristics are shown in Table 1. No significant differences were observed in baseline characteristics. In the BVS group, more intravascular imaging was used during the procedure. The types of mDES implanted were Xience in 26.6%, Resolute in 23.8%, Synergy in 22.4%, Orsiro in 12.5%, Biomatrix in 9.7%, and others in 5%.
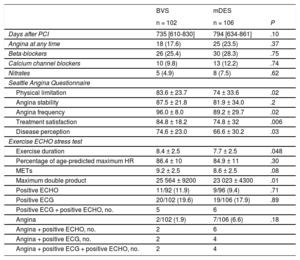
The symptomatic evaluation by means of the SAQ and the results of the noninvasive stress tests are shown in Table 2. Angina at any time was reported in 18 patients (17.6%) of the BVS group and 25 (23.5%) of the mDES group (P = .37). The SAQ showed significantly better results for the BVS group. In 188 patients (90.3%) ECHO was properly obtained in the exercise test with good quality of imaging. The level of stress achieved in the BVS group was higher regarding exercise duration and peak double product. Ex-ECHO was positive in 11/92 patients (11.9%) in the BVS group and 9/96 patients (9.4%) in the mDES group (P = .71); ECG was positive in 20/102 patients (19.6%) vs 19/106 (17.9%) (P = .89) and angina was provoked in 2/102 (1.9%) vs 7/106 (6.6%) (P = .18), respectively. In both groups, ischemic tests were more frequently positive in ECG than in ECHO. What seemed to be different was the frequency of clinically positive results. In mDES, almost all patients with ischemia also showed angina but, in the BVS group, angina was elicited in a minority of the patients showing ischemia. The level of exercise achieved was also greater in the BVS group among those patients reporting recurrent angina in follow-up and among those with positive ECHO or ECG tests (Table 3).
Clinical Evaluation and Stress Tests Results
| BVS | mDES | ||
|---|---|---|---|
| n = 102 | n = 106 | P | |
| Days after PCI | 735 [610-830] | 794 [634-861] | .10 |
| Angina at any time | 18 (17.6) | 25 (23.5) | .37 |
| Beta-blockers | 26 (25.4) | 30 (28.3) | .75 |
| Calcium channel blockers | 10 (9.8) | 13 (12.2) | .74 |
| Nitrates | 5 (4.9) | 8 (7.5) | .62 |
| Seattle Angina Questionnaire | |||
| Physical limitation | 83.6 ± 23.7 | 74 ± 33.6 | .02 |
| Angina stability | 87.5 ± 21.8 | 81.9 ± 34.0 | .2 |
| Angina frequency | 96.0 ± 8.0 | 89.2 ± 29.7 | .02 |
| Treatment satisfaction | 84.8 ± 18.2 | 74.8 ± 32 | .006 |
| Disease perception | 74.6 ± 23.0 | 66.6 ± 30.2 | .03 |
| Exercise ECHO stress test | |||
| Exercise duration | 8.4 ± 2.5 | 7.7 ± 2.5 | .048 |
| Percentage of age-predicted maximum HR | 86.4 ± 10 | 84.9 ± 11 | .30 |
| METs | 9.2 ± 2.5 | 8.6 ± 2.5 | .08 |
| Maximum double product | 25 564 ± 9200 | 23 023 ± 4300 | .01 |
| Positive ECHO | 11/92 (11.9) | 9/96 (9.4) | .71 |
| Positive ECG | 20/102 (19.6) | 19/106 (17.9) | .89 |
| Positive ECG + positive ECHO, no. | 5 | 6 | |
| Angina | 2/102 (1.9) | 7/106 (6.6) | .18 |
| Angina + positive ECHO, no. | 2 | 6 | |
| Angina + positive ECG, no. | 2 | 4 | |
| Angina + positive ECG + positive ECHO, no. | 2 | 4 | |
BVS, bioresorbable vascular scaffold; ECHO, echocardiography; ECG, electrocardiography; HR, heart rate; mDES, metallic drug-eluting stent; METs, metabolic equivalent units; PCI, percutaneous coronary intervention.
Unless otherwise indicated, values are expressed as no. (%), no./No. (%), mean ± standard deviation or median [interquartile range].
Performance in Exercise Tests for Patients With Recurrent Angina and Positive Stress Tests
| BVS | mDES | P | |
|---|---|---|---|
| Recurrent angina, no. | 18 | 25 | |
| Exercise duration, min | 7.5 ± 1.2 | 6.6 ± 3.0 | .23 |
| Percentage of age-predicted maximum HR | 83.8 ± 12 | 74.5 ± 16 | .04 |
| METs | 8.5 ± 1.4 | 8 ± 2.7 | .47 |
| Maximum double product | 21 643 ± 5687 | 19 774 ± 2464 | .15 |
| Positive ECG or ECHO tests, no. | 26 | 22 | |
| Exercise duration, min | 9.0 ± 2.0 | 7.7 ± 1.8 | .02 |
| Percentage of age-predicted maximum HR | 90.5 ± 9.0 | 87.8 ± 5.4 | .24 |
| METs | 9.9 ± 2.0 | 9.2 ± 1.8 | .21 |
| Maximum double product | 24 727 ± 11 030 | 21 040 ± 2979 | .12 |
BVS, bioresorbable vascular scaffold; ECHO, echocardiography; ECG, electrocardiography; HR, heart rate; mDES, metallic drug-eluting stent; METs, metabolic equivalent units.
Unless otherwise indicated, values are expressed as mean ± standard deviation.
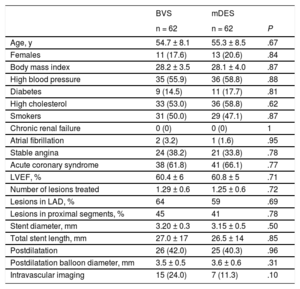
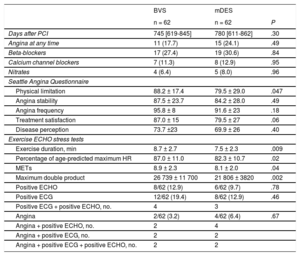
A propensity score matching was applied to the cohort with stress ECHO, yielding 62 pairs of patients. The estimated postmatching standardized differences for all covariates were all < 10%, indicating an adequate balance between the 2 groups. In fact, both groups showed a fairly similar profile with no significant differences (Table 4). Angina at any time was found in 11 patients (17.7%) in the BVS group vs 15 patients (24.1%) in the mDES group (P = .49) and SAQ showed significantly better results for the BVS group in physical limitations with a strong trend in treatment satisfaction (Table 5). The stress test performed was higher as measured by all parameters in the BVS group. Ex-ECHO was positive in 8/62 (12.9%) patients in the BVS group and in 6/62 (9.7%) patients in the mDES group (P = .78), the ECG test was positive in 12/62 (19.4%) vs 8/62 (12.9%) (P = .46) and angina was present in 2/62 (3.2%) vs 4/62 (6.4%) (P = .67), respectively (Table 5).
Baseline Characteristics in Matched Groups
| BVS | mDES | ||
|---|---|---|---|
| n = 62 | n = 62 | P | |
| Age, y | 54.7 ± 8.1 | 55.3 ± 8.5 | .67 |
| Females | 11 (17.6) | 13 (20.6) | .84 |
| Body mass index | 28.2 ± 3.5 | 28.1 ± 4.0 | .87 |
| High blood pressure | 35 (55.9) | 36 (58.8) | .88 |
| Diabetes | 9 (14.5) | 11 (17.7) | .81 |
| High cholesterol | 33 (53.0) | 36 (58.8) | .62 |
| Smokers | 31 (50.0) | 29 (47.1) | .87 |
| Chronic renal failure | 0 (0) | 0 (0) | 1 |
| Atrial fibrillation | 2 (3.2) | 1 (1.6) | .95 |
| Stable angina | 24 (38.2) | 21 (33.8) | .78 |
| Acute coronary syndrome | 38 (61.8) | 41 (66.1) | .77 |
| LVEF, % | 60.4 ± 6 | 60.8 ± 5 | .71 |
| Number of lesions treated | 1.29 ± 0.6 | 1.25 ± 0.6 | .72 |
| Lesions in LAD, % | 64 | 59 | .69 |
| Lesions in proximal segments, % | 45 | 41 | .78 |
| Stent diameter, mm | 3.20 ± 0.3 | 3.15 ± 0.5 | .50 |
| Total stent length, mm | 27.0 ± 17 | 26.5 ± 14 | .85 |
| Postdilatation | 26 (42.0) | 25 (40.3) | .96 |
| Postdilatation balloon diameter, mm | 3.5 ± 0.5 | 3.6 ± 0.6 | .31 |
| Intravascular imaging | 15 (24.0) | 7 (11.3) | .10 |
BVS, bioresorbable vascular scaffold; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; mDES, metallic drug-eluting stent.
Unless otherwise indicated, values are expressed as no. (%) or mean ± standard deviation.
Clinical Evaluation and Ischemic Tests Results in Matched Groups
| BVS | mDES | ||
|---|---|---|---|
| n = 62 | n = 62 | P | |
| Days after PCI | 745 [619-845] | 780 [611-862] | .30 |
| Angina at any time | 11 (17.7) | 15 (24.1) | .49 |
| Beta-blockers | 17 (27.4) | 19 (30.6) | .84 |
| Calcium channel blockers | 7 (11.3) | 8 (12.9) | .95 |
| Nitrates | 4 (6.4) | 5 (8.0) | .96 |
| Seattle Angina Questionnaire | |||
| Physical limitation | 88.2 ± 17.4 | 79.5 ± 29.0 | .047 |
| Angina stability | 87.5 ± 23.7 | 84.2 ± 28.0 | .49 |
| Angina frequency | 95.8 ± 8 | 91.6 ± 23 | .18 |
| Treatment satisfaction | 87.0 ± 15 | 79.5 ± 27 | .06 |
| Disease perception | 73.7 ±23 | 69.9 ± 26 | .40 |
| Exercise ECHO stress tests | |||
| Exercise duration, min | 8.7 ± 2.7 | 7.5 ± 2.3 | .009 |
| Percentage of age-predicted maximum HR | 87.0 ± 11.0 | 82.3 ± 10.7 | .02 |
| METs | 8.9 ± 2.3 | 8.1 ± 2.0 | .04 |
| Maximum double product | 26 739 ± 11 700 | 21 806 ± 3820 | .002 |
| Positive ECHO | 8/62 (12.9) | 6/62 (9.7) | .78 |
| Positive ECG | 12/62 (19.4) | 8/62 (12.9) | .46 |
| Positive ECG + positive ECHO, no. | 4 | 3 | |
| Angina | 2/62 (3.2) | 4/62 (6.4) | .67 |
| Angina + positive ECHO, no. | 2 | 4 | |
| Angina + positive ECG, no. | 2 | 2 | |
| Angina + positive ECG + positive ECHO, no. | 2 | 2 | |
BVS, bioresorbable vascular scaffold; ECHO, echocardiography; ECG, electrocardiography; HR, heart rate; mDES, metallic drug-eluting stent; METs, metabolic equivalent units; PCI, percutaneous coronary intervention.
Unless otherwise indicated, values are expressed as no. (%), no./No. (%), mean ± standard deviation or median [interquartile range].
As in the overall analysis, in this matched cohort, ischemic tests were more frequently positive in ECG than in ECHO and these positive results were more frequently associated with angina in patients treated with mDES than in those treated with BVS. Cardiac performance on exercise tended to be higher in the BVS group among those patients reporting recurrent angina in follow-up and among those with positive ECHO or ECG tests (Table 6).
Performance in Exercise Tests for Matched Patients With Recurrent Angina and Positive Stress Tests
| BVS | mDES | P | |
|---|---|---|---|
| Recurrent angina, no. | 11 | 15 | |
| Exercise duration, min | 8.0 ± 2.6 | 6.5 ± 2.9 | .19 |
| Percentage of age-predicted maximum HR | 82 ± 12 | 79 ± 15 | .59 |
| METs | 9.3 ± 2.7 | 8.0 ± 2.7 | .31 |
| Maximum double product | 21 853 ± 2687 | 19 886 ± 2972 | .20 |
| Positive ECG or ECHO tests, no. | 16 | 11 | |
| Exercise duration, min | 9.4 ± 2.7 | 8.5 ± 1.2 | .33 |
| Percentage of age-predicted maximum HR | 93.0 ± 6 | 87.6 ± 6 | .03 |
| METs | 9.9 ± 2.7 | 9.2 ± 1.8 | .47 |
| Maximum double product | 25 811 ± 4536 | 21 842 ± 2979 | .21 |
BVS, bioresorbable vascular scaffold; ECHO, echocardiography; ECG, electrocardiography; HR, heart rate; mDES, metallic drug-eluting stent; METs, metabolic equivalent units.
Unless otherwise indicated, values are expressed as mean ± standard deviation.
The primary endpoint of the study was not met, since a comparable rate of positive results in stress ECHO was found. The main findings of this study are the following: a) rates of angina reported at any time in the 2-year follow-up were equivalent in both groups; b) ischemia detection in ECHO and ECG during stress was comparable in both groups; c) the results of the SAQ were partially more satisfactory for the BVS group; d) patients treated with BVS developed higher stress on exercise; e) recurrent angina rates during follow-up clearly exceeded the rates of positive clinical and ECHO results on stress tests.
Bioresorbable vascular scaffold may theoretically offer potential advantages over mDES. The release of vessel from a metallic cage could help in the restoration of physiological vasomotion, mechanotransduction, adaptive shear stress, late luminal gain, and late expansive remodeling.4–12,20,21 However, conflicting results have been reported regarding vasomotion since, in the ABSORB II trial, the Absorb bioresorbable scaffold did not show superior vasomotor reactivity compared with the metallic stent. In fact, the overall vasodilation of the scaffold was very similar to that observed in previous studies,22,23 but surprisingly, the assumption of no vasodilation for the metallic stent, as previously reported,24–26 was not met. In view of these unexpected results, it has been suggested that future trials should consider alternative imaging, different vasodilator responses, or later follow-up to confirm whether there is a true vasomotor advantage for the bioresorbable scaffold or whether this proposed benefit does not occur in practice when compared with contemporary mDES.14
Therefore, it is quite pertinent to evaluate whether all those potential advantages of the BVS on vascular function could translate into a clinical benefit for patients.
In the ABSORB II trial, no differences in SAQ or exercise test results were observed at 1 and 3 years.13,14 Regarding recurrent angina, in a post hoc analysis, the cumulative angina rates at 1 year were lower in the bioresorbable scaffold group than in the metallic stent group (22% vs 30%; P = .04),13 but these were similar at 3 years.14 In a recent subanalysis of the ABSORB II trial, the investigators reported that patients with site-reported angina showed higher rates of cardiovascular events, cardiac resource use, and positive exercise tests than those without site-reported angina.27 On the other hand, in the larger ABSORB III trial, the 1-year rates of patient reported angina were also nearly identical with the 2 devices.15
Both ABSORB II and III are randomized studies not showing an advantage of BVS over mDES in terms of symptomatic status. However, there are concerns that should be raised with respect to the methodology. First, in the ABSORB III trial, recurrent angina was reported 12 months after implantation, but it is plausible that scaffold resorption takes longer to fully restore vessel functionality. Second, the noninvasive tests for ischemia performed in ABSORB II consisted of exercise tests with ECG recording, which clearly offers lower diagnostic accuracy for ischemia than stress ECHO. Third, these trials were not specifically designed to assess angina and ischemia in stress tests and the inclusion/exclusion criteria were not aimed to specifically assess these objectives.
In our study, all patients had an uneventful follow-up at 24 ± 6 months. This time of follow-up allows further resorption of the BVS. Although strut remnants may be visualized at this time point, the mechanical integrity of the scaffold is supposed to be mostly lost. This could theoretically promote a more adequate restoration of vascular function or even a certain degree of expansive remodeling, especially in those with moderate amount of neointimal proliferation. On the other hand, this time frame is long enough to exclude most patients with significant restenosis but is not so long as to introduce a relevant prevalence of disease progression. Given that all patients underwent complete revascularization according to the inclusion criteria, the influence of underlying restenosis, residual disease, and lesion progression on the findings could have been diminished. Additionally, with respect to restenosis, the 2 the above-mentioned trials have shown a similar incidence of target lesion and target vessel revascularization at 12 months with BVS and metallic everolimus-eluting stents,13–15 and angiographic surveillance has confirmed a low restenosis rate for BVS.28
Of note, for the purpose of this study, patients were carefully selected through a number of criteria to ensure adequate interpretation of symptoms and stress tests results and to have comparable groups of patients treated with BVS and mDES. After this selection, no significant differences were observed in most of the variables, but there was a certain trend for a more adverse profile in the mDES group, reflecting the trends operating in real practice for the selection between BVS or mDES. In addition, a higher use of intravascular imaging along with a trend for wider and shorter devices and more postdilatation were observed with BVS, which could influence the results. Thus, a propensity score matching was performed, rendering groups with even more similar characteristics. Therefore, the differences in symptomatic status and stress test results found in this study could be attributed mostly to the different stent models.
The rate of angina reported at any time was comparable in both groups, as was the detection of ischemia in stress tests, either in ECG or in ECHO (less common). However some differences between groups were observed in functional parameters. The SAQ results were partially better for patients with BVS, which means that the frequency, intensity, and limitation imposed by recurrent angina was lower in patients treated with BVS. On the other hand, although the detection of ischemia in stress ECHO tests were similar between groups, the magnitude of stress reached in exercise testing was greater in patients with BVS and the association of angina with ischemia in tests was less frequent in these patients.
The discrepancy between a similar rate of recurrent angina and the different results in SAQ could be explained mostly by the different meaning of the 2 endpoints but also by the different metrics. The SAQ represents assessment of a 4-week window (recall period) and does not fully take into account earlier episodes of angina. Recurrent angina at any time during a 2-year follow-up may account for a certain variety of symptoms, from a single episode to daily symptoms, and does not take into consideration the limitations imposed on physical activities and quality of life.
Of interest, rates of recurrent angina clearly exceeded the rates of positive results in stress tests, especially those of provoked angina. This observation is in agreement with findings in ABSORB II, in which rates of recurrent angina, positive ECG tests, and clinically positive tests were in the range of our study.13,14 Anginal episodes during follow-up are related to both anatomical substrates and a myriad of dynamic dysfunctional factors operating in conjunction, whereas stress tests performed at a specific moment do not take all these factors into account. Another plausible explanation could be the consideration as angina recurrence of episodes of chest pain that could correspond to other causes, especially those with more atypical characteristics for angina.
The results of our study do not provide conclusive support for the potential advantages of BVS over mDES in terms of angina and ischemia during follow-up. The differences found in some aspects of the SAQ and in exercise performance should be interpreted very cautiously and warrant further investigation.
A similar design should be applied to evaluate patients with multivessel interventions or very long stenting (“full polymer vs full metal jacket”) in which the potential functional advantage of BVS (if any) could be more easily identified. Properly designed randomized trials in general and selected populations are warranted.
LimitationsThe main limitation of this study is its nonrandomized nature and therefore the results should be interpreted with caution. The numerous inclusion/exclusion criteria applied allowed a reasonable degree of matching and prevented the presence of many confounding factors, and the additional propensity score matching provided fairly comparable groups. In this regard, the strict criteria applied could be seen as a limitation for applicability of the results, but this is a mechanistic study specifically designed to address the objective of residual ischemia related to the stented segment only. On the other hand, we acknowledge that the necessary exclusion of patients with clinical restenosis could bias the perception of the overall effect of BVS on the endpoint of angina. However, the change of this bias is decreased by the fairly comparable rates of target lesion revascularization with mDES and BVS reported in trials.13–15
One of the limitations of this study is that the patients were not blinded to treatment allocation. Therefore, differences in SAQ could be explained to some extent by the assumption that the patients perceived that they received better treatment with BVS. However, the SAQ is not so much a quality of life test as an inquiry on angina status. In this regard, patients are unlikely to deny or hide angina episodes from their physicians, being aware of the potential implications. In addition, functional status was objectively assessed by treadmill tests conducted by blinded investigators.
The SAQ represents assessment of a 4-week window (recall period) and does not fully take into account earlier episodes of angina. Although all patients were asked about the occurrence of angina episodes throughout the time period after the procedure and until the application of the questionnaires, the time and duration of angina was not recorded through adverse event reporting because of the study design. For the same reason, the SAQ were not applied before revascularization. The sample size was not large and consequently the statistic power is limited, which could potentially explain the negative results. The strict and numerous selection criteria, the required length of follow-up, and the imaging technique applied limited the number of patients that could be included.
CONCLUSIONSThe primary endpoint of the study (positive stress ECHO) was comparable in the 2 groups. In addition, recurrent angina and the presence of angina/ischemia in stress tests were similar in patients treated with BVS or mDES. Future studies should confirm the better functional status, assessed by means of the SAQ and exercise performance, detected in patients receiving BVS.
CONFLICTS OF INTERESTNone declared.
- –
The rationale supporting the design of bioresorbable scaffolds is to overcome the limitations associated with the permanent presence of a metallic cage in the vessel. These include impaired vasomotion, no possible expansive-adaptive remodeling in the presence of in-stent plaque growth, thrombogenicity of uncovered struts, and induced neoatherosclerosis.
- –
The theorical restoration of vasomotion allowed by these devices could be translated into a lower incidence of angina and ischemia under stress conditions, unrelated to restenosis or new lesions. However, the clinical findings in this regard have been inconclusive so far.
- –
This is the first study specifically designed to evaluate angina and ischemia in stress ECHO in the long-term in matched patients treated with bioresorbable and mDES. This is not a randomized trial, but the inclusion/exclusion criteria applied allowed a reasonable degree of matching and prevented the presence of many confounding factors. In addition, the propensity score matching provided even more similar groups.
- –
The primary endpoint was similar in the 2 groups and recurrent angina showed no differences. However functional status was better in patients receiving bioresorbable scaffolds. Nevertheless, these results are not conclusive and the potential benefits of bioresorbable scaffolds should be confirmed in further research.