Although the incidence of cardiovascular diseases classically associated with human immunodeficiency virus (HIV) has decreased considerably with antiretroviral therapy, cardiovascular risk, and especially ischemic heart disease, are higher in HIV-infected patients than in uninfected individuals. This is due to the interaction of patient-dependent factors with virus-dependent factors, as well as factors associated with antiretroviral therapy. With increasing of life expectancy and the chronicity of HIV infection, cardiovascular disease has emerged as an important cause of morbidity and mortality in HIV patients. In developed countries, the most common cardiovascular manifestation of HIV is ischemic heart disease. Currently, it is not uncommon to find HIV patients with acute coronary syndrome and, given the important pharmacokinetic interactions of antiretroviral drugs, it is important to know which cardiovascular treatments are safe in this group of patients. The ideal approach would be to mitigate the cardiovascular risk in HIV patients with specific primary prevention measures. All these issues are discussed in this review, which aims to aid clinical cardiologists faced with HIV patients with ischemic heart disease or with high cardiovascular risk in daily clinical practice.
Keywords
The problem of heart disease associated with human immunodeficiency virus (HIV) has been addressed in a general way by Boccara and Cohen in Revista Española de Cardiología.1 These authors highlight the increased cardiovascular (CV) risk in the HIV population compared with that in the general population. In this review, we take an in-depth look at the mechanisms and proper assessment of CV risk in HIV patients, with special emphasis on the drug interactions of antiretroviral therapy with medication used in primary and secondary CV prevention, an aspect that we consider essential in the CV risk management of HIV patients.
CARDIOVASCULAR DISEASE AS A CAUSE OF DEATH IN HIV PATIENTSThe HIV infection and the most advanced stage of infection–acquired immunodeficiency syndrome (AIDS)–have a global health and social impact and continue to be a challenge for the health system.2 Data from the United Nations show that there are currently more than 35 million people worldwide living with HIV, with 10 new cases being diagnosed in Spain 10 daily.3
The HIV era officially began on June 5, 1981, when the Center for Disease Control and Prevention convened a press conference that presented 5 cases of pneumonia due to Pneumocystis carinii (now called Pneumocystis jiroveci). In the following months, several cases of Kaposi sarcoma were found. Although physicians were familiar with both pneumonia due to P. carinii and Kaposi sarcoma, the joint appearance of both entities in several patients grabbed their attention. Most of those patients were sexually active gay men, many of whom also had other chronic diseases later identified as opportunistic infections. Blood tests showed that these patients did not have an adequate number of a type of blood cell called CD4 T-lymphocytes. Most of these patients died within a few months. The era of antiretroviral therapy began 5 years later, with the first clinical trial with zidovudine in 1986. New antiretroviral drugs were subsequently developed, but for the first 10 years this antiretroviral therapy was ineffective. Highly active antiretroviral therapy (HAART) was implemented in 1996. Before the introduction of HAART, mortality was 20 cases per 100 000 per year. After HAART, it was reduced to 2 cases per 100 000 per year.4
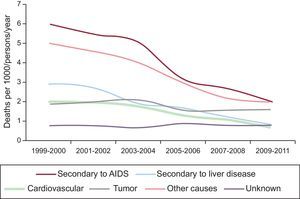
On the basis of the Data Collection on Adverse Effect of Anti-HIV Drugs (DAD) study,5 which included HIV-infected patients receiving HAART from March 1999 to February 2011 from different continents, the most common causes of death in HIV patients (Figure 1) were AIDS, followed by cancer, liver disease, and CV diseases in fourth place (11% of total mortality). Within this group, acute myocardial infarction (AMI) accounted for 54.5% of total CV mortality. In that study, mortality, especially mortality associated with AIDS, progressively decreased, primarily due to the widespread use of increasingly effective antiretroviral therapy in developed countries. With HAART use, life expectancy has increased considerably, to the point that it is approaching that in the general population. Cardiovascular mortality also decreased significantly in HIV patients during the DAD study period, with a decrease of over 65% from 1999 to 2011. While deaths from CV causes in the general population also declined in this period, the decline was even greater in HIV patients. This reduction is not explained only by HAART itself; it is also a result of increased screening and early management of CV risk factors, as well as increased preventive measures during the study period (eg, tobacco cessation, diet, exercise, lipid-lowering therapy), resulting in a reduction of the incidence of CV disease. Therefore, in developed countries, morbidity and mortality in HIV-infected patients no longer depend exclusively on the typical manifestations of AIDS (such as opportunistic diseases), as was the case in the early years, but rather CV disease has emerged as a relevant cause of death.6
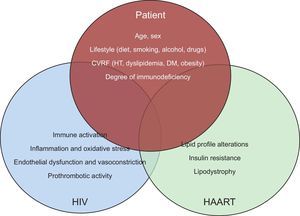
PATHOGENESIS OF CARDIOVASCULAR DISEASE IN HIV PATIENTSCardiovascular risk is increased in HIV-infected patients. This risk is due to: a) factors intrinsic to the patient, such as the higher prevalence of classic CV risk factors, b) factors associated with HIV itself, such as immune activation, inflammation, and immunodeficiency, and c) factors associated with antiretroviral therapy, primarily through its metabolic alterations (Figure 2).7
Interaction between patient characteristics, HIV infection and antiretroviral therapy in the development of cardiovascular disease. CVRF, cardiovascular risk factors; DM, diabetes mellitus, HAART, highly active antiretroviral therapy; HIV, human immunodeficiency virus; HT, hypertension.
- •
Age is associated with progressive organ dysfunction. With greater life expectancy and the chronicity of the infection, HIV patients have a greater propensity to develop CV disease, which was previously limited by their shorter life expectancy due to the HIV infection per se.
- •
Male sex has been shown to increase the risk of CV disease, based primarily on the protective role of estrogen during women's fertile period. Since HIV infection is more common in men, this leads to an increased CV risk compared with that the general population.
- •
Drug use, whose incidence is higher HIV patients than in the general population, has been associated with an increased risk of CV disease.
- •
HIV-infected patients have higher blood pressure levels than the general population. This is believed to be largely due to the effect of HIV itself, as well as to antiretroviral therapy, although no antiretroviral drugs have been identified as causative agents.
- •
The incidence of dyslipidemia is also increased in HIV-infected patients, mainly in connection with HAART. These issues are discussed with more detail in the following sections.
- •
The incidence of insulin resistance and diabetes mellitus (DM) has also increased in patients exposed to HAART.
- •
HIV patients show changes in the distribution of body fat, partly because of all the above-mentioned metabolic abnormalities. Lipodystrophy, which is an abnormal accumulation of body fat, predominantly at the abdominal and visceral levels with loss of fat in cheeks and limbs, is typical of HIV patients and is secondary to some antiretroviral agents, and is associated with an increased risk of CV disease.
Thus, HIV patients show a higher prevalence of traditional CV risk factors.8 One of the subanalyses of the DAD study studied the role of traditional CV risk factors in HIV patients with AMI and HAART. Age, male sex, smoking, hypertension, DM, dyslipidemia, and having a moderate to high Framingham score were associated with an increased risk of AMI in HIV patients compared with the general population.9
Contribution of HIV InfectionAfter adjustment for traditional CV risk factors, HIV-infected patients have a 50% higher risk of CV disease than that in uninfected persons.10 This risk is markedly increased with fewer CD4 cells. The role of HIV in CV disease is based on several factors:
- •
First, viral replication stimulates immune activation. It has been postulated that increased levels of CD4- and CD8-activated lymphocytes are associated with a greater degree of endothelial dysfunction and an increased rate of atherothrombotic events. However, several studies on this topic have shown inconsistent results. With regard to innate immunity, a relationship has recently been found between activation of monocytes/macrophages and atherosclerosis measured by carotid artery intima-media thickness.11,12
- •
A recent study showed an association between cytomegalovirus infection in asymptomatic HIV patients with increased atherosclerosis and subclinical CV disease. Similar results were obtained with herpes simplex virus type 2 infection and also with hepatitis C virus infection.13
- •
HIV promotes vasoconstriction, inflammation, and endothelial dysfunction through the TAT protein. Thus, HIV promotes lipid deposition and atherosclerotic plaque development.14
- •
Finally, perhaps the most striking argument is that inflammation secondary to infection plays a major role in the development of atherosclerosis. Inflammation affects endothelial cells and promotes a hypercoagulable state leading to atherosclerosis and plaques rupture.15
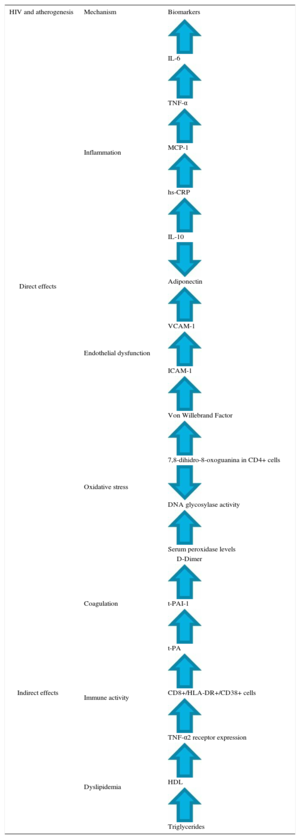
Table 1 summarizes the different altered biomarkers in clinical trials of HIV patients without antiretroviral treatment. HIV causes a dysregulation of coagulation, with increased fibrinolysis markers such as D-dimer or von Willebrand factor.16 In this line, it has been found that HAART decreases the degree of inflammation and immune activation. However, both processes remain at higher levels in HIV patients than in the general population, and therefore CV risk also remains higher.17
Biomarkers in HIV Patients Without Antiretroviral Therapy and Their Role in Atherosclerosis
| HIV and atherogenesis | Mechanism | Biomarkers |
|---|---|---|
| Direct effects | Inflammation | IL-6 |
| TNF-α | ||
| MCP-1 | ||
| hs-CRP | ||
| IL-10 | ||
| Adiponectin | ||
| Endothelial dysfunction | VCAM-1 | |
| ICAM-1 | ||
| Von Willebrand Factor | ||
| Oxidative stress | 7,8-dihidro-8-oxoguanina in CD4+ cells | |
| DNA glycosylase activity | ||
| Serum peroxidase levels | ||
| Indirect effects | Coagulation | D-Dimer |
| t-PAI-1 | ||
| t-PA | ||
| Immune activity | CD8+/HLA-DR+/CD38+ cells | |
| TNF-α2 receptor expression | ||
| Dyslipidemia | HDL | |
| Triglycerides |
HDL, high-density lipoprotein; HLA-DR, human leukocyte antigen-antigen D related; hs-CRP: high-sensitivity C reactive protein; ICAM, intercellular adhesion molecule; IL, interleukin; MCP-1, monocyte chemoattractant protein-1; TNF, tumor necrosis factor; t-PA, tissue plasminogen activator; t-PAI-1, tissue-type plasminogen activator inhibitor 1; VCAM, vascular cell adhesion molecule.
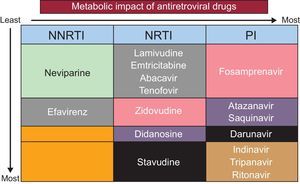
Antiretroviral therapy is associated with an increased risk of CV disease in HIV patients, although the mechanism is not fully understood. Each antiretroviral drugs differs in its contribution to CV risk, mainly producing lipid and metabolic disorders, such as insulin resistance (Figure 3).18,19
Currently, there are 6 therapeutic groups of antiretroviral drugs in Spain, with different mechanisms of action, sometimes allowing their combination to enhance their therapeutic actions. Nucleoside analog reverse transcriptase inhibitors and nonnucleoside reverse transcriptase inhibitors (NNRTIs) slow the enzyme reverse transcriptase, which is the enzyme used by HIV to change its RNA into viral DNA and prevent the creation of new genetic material; in this way, HIV cannot take over the command center of the CD4 cell. Protease inhibitors (PIs) prevent the assembly of new viruses, stopping the protease enzyme used by HIV to cut and assemble new virus particles. Although there have been new viral particles, they cannot be assembled correctly, and the defective virus cannot infect other cells. Fusion inhibitors act in the first stage of the HIV replication cycle, blocking HIV at a completely different stage of the HIV life cycle than other drugs. This is very important for the treatment of people who have developed resistance to other drugs or who cannot tolerate them.
Table 2 summarizes the main drugs used in HAART, with their more common metabolic and CV adverse effects.20 The preferred treatment is usually a combination of 2 nucleoside analog reverse transcriptase inhibitors (usually tenofovir and emtricitabine, although the association of abacavir and lamivudine is also common) with an integrase inhibitor (eg, dolutegravir or elvitegravir boosted with cobicistat), or an IP (eg, darunavir or ritonavir), or an NNRTI (eg, rilpivirine or efavirenz), although there are other alternative combinations.21
Antiretroviral Drugs and Their Cardiovascular Risk
| Protease inhibitors | |
| Atazanavir | Dyslipidemia, decreased glomerular filtration rate |
| Darunavir | Dyslipidemia |
| Fosamprenavir | Dyslipidemia |
| Indinavir | Dyslipidemia, diabetes mellitus |
| Lopinavir | Dyslipidemia, decreased glomerular filtration |
| Saquinavir | Dyslipidemia |
| Tipranavir | Dyslipidemia, intracranial bleeding |
| Nonnucleoside reverse transcriptase inhibitors | |
| Efavirenz | Dyslipidemia |
| Etravirine | |
| Neviparine | |
| Rilpivirine | Decreased glomerular filtration |
| CCR5 inhibitors | |
| Maraviroc | Ischemic heart disease |
| Integrase inhibitors | |
| Dolutegravir | Decreased glomerular filtration |
| Elvitegravir | Decreased glomerular filtration |
| Raltegravir | |
| Nucleoside reverse transcriptase inhibitors | |
| Abacavir | Ischemic heart disease |
| Stavudine | Dyslipidemia |
| Didanosine | Ischemic heart disease, dyslipidemia |
| Emtricitabine | |
| Lamivudine | |
| Tenofovir | Decreased glomerular filtration |
| Zidovudine | Dyslipidemia |
| Fusion inhibitors | |
| Enfuvirtide | |
| Enhancers | |
| Cobicistat | Decreased glomerular filtration |
| Ritonavir | Decreased glomerular filtration |
Although HAART cannot eradicate HIV infection, viral replication remains suppressed, restoring and preserving the immune status by increasing the number of CD4 lymphocytes. In this way, HAART reduces HIV-related morbidity and mortality and other comorbidities and improves quality of life. The HAART administration is currently recommended in all patients with HIV infection regardless of CD4 levels, although the strength of the evidence varies depending on CD4 count. The SMART study showed that antiretroviral therapy should be prescribed indefinitely, except in the case of certain incidents.22,23
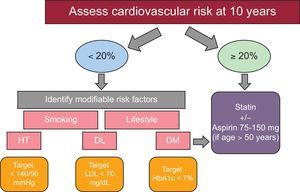
CARDIOVASCULAR DISEASE PREVENTIONAccording to the latest clinical practice guidelines of the European Society of AIDS,21 annual monitoring focusing on traditional CV risk factors (hypertension, DM, dyslipidemia) is recommended, with assessment of changes in body composition and the performance of electrocardiogram. It is mandatory to assess CV risk at diagnosis of HIV infection and before the introduction of HAART. CV risk assessment is not indicated for routine echocardiography in asymptomatic HIV patients. In addition, CV risk assessment with the Framingham score is recommended in all men older than 40 years and in women older than 50 years without CV disease. Although the usefulness of the Framingham score in HIV patients is suboptimal because it tends to underestimate the risk of CV disease, it is reasonable to use it for a first assessment of CV risk. An online risk calculator to estimate CV risk with the Framingham score, as well as with the EuroSIDA and DAD-risk scores, is available at the HIV pharmacovigilance website.24,25 In all patients with high CV risk (> 20%), adjustment of HAART is recommended (eg, consider replacing zidovudine or abacavir with tenofovir, or use a therapy without NNRTIs), in addition to strict adherence to CV therapeutic objectives,20,26,27 with appropriate lifestyle interventions (Table 3).
Lifestyle Interventions in HIV Patients
| Dietary counselling | • Dietary intervention should not interfere with the dietary requirements necessary for appropriate absorption of antiretroviral drugs |
| • Keep caloric intake balanced with energy expenditure | |
| • Limit intake of saturated fat, cholesterol, and refined carbohydrates | |
| • Reduce total fat intake to < 30% and dietary cholesterol to < 300 mg/d | |
| • Emphasize intake of vegetables, fruit and grain products with fiber | |
| • Cut back on beverages and foods with added sugar | |
| • Choose and prepare foods with little or no salt. Aim to eat < 1500mg of sodium per day | |
| • Emphasize consumption of fish, poultry (without skin) and lean meat | |
| • Consider referral to dietician, 1-week food and drink diary to discover ‘hidden’ calories | |
| • Avoid binge eating (‘yo-yo dieting’) | |
| • In persons with HIV-related wasting and dyslipidemia, address wasting first and consider referral to dietician | |
| • Persons who are obviously overweight should be encouraged to lose weight. Starvation diets are not recommended (immune defense mechanisms potentially decreased) Malnutrition has to be addressed when observed | |
| • The following questions are helpful to determine average alcohol intake | |
| • Intake of alcohol should be restricted to no more than 1 drink per day for women and 2 drinks per day for men (< 20-40 g/day). | |
| • In particular, persons with liver disease, adherence problems, inadequate CD4 cell increase, tumors, past tuberculosis, diarrhea and other conditions associated with high alcohol intake should be encouraged to decrease or stop alcohol intake | |
| Exercise promotion | • Promote an active lifestyle to prevent and treat obesity, hypertension, and diabetes |
| • Encourage self-directed moderate level physical activity (eg, taking the stairs, cycling or walking to work, cycling, swimming, hiking) | |
| • Emphasize regular moderate-intensity exercise rather than vigorous exercise | |
| • Achieve cardiovascular fitness (eg, 30min of brisk walking > 5 days a week) | |
| • Maintain muscular strength and joint flexibility |
HIV, human immunodeficiency virus.
Figure 4 shows a scheme for the prevention of CV disease. Drug therapy to treat traditional CV factors is reserved for certain subgroups in which the potential benefits exceed the adverse effects. Some people benefit from several combined interventions. For each 10mmHg reduction in systolic pressure, each reduction of 39mg/dL (1 mmol/L) in total cholesterol, and for the use of aspirin, the risk of ischemic heart disease is reduced by 20% to 25% with each intervention, this effect being additive.28,29 Observational studies suggest that smoking cessation decreases the risk of ischemic heart disease by approximately 50%, and this effect is also additive to other interventions.30
The incidence of DM is 4 times higher in HIV patients than in the general population.31 Given the increased risk of insulin resistance, glycated hemoglobin measurement is recommended at initiation HAART, and between 4 and 12 weeks after the start of a new treatment, and every 3-6 months with stable HAART. Of importance, glycated hemoglobin values in HIV-infected patients receiving antiretroviral therapy, especially with abacavir, tend to underestimate type 2 diabetes. In addition, prediabetic status is also important, as both glucose intolerance and impaired fasting glucose increase morbidity and CV mortality and raise the risk of developing DM by 4- to 6-fold. These people should be advised to change their lifestyle and to assess and treat CV risk factors. For treatment, the same therapeutic algorithm is recommended as in the non-HIV population, the first recommended drug option being metformin (it is important to bear in mind that it can worsen lipoatrophy). Insulin is the therapy of choice when there is no response to other antidiabetic drugs. The remaining therapeutic options are considered as a last therapeutic step because of the lack of clinical trials with HIV-infected patients.
Both the HIV virus and its treatment can induce dyslipidemia, increasing the frequency of patterns with low high-density lipoprotein, and high low-density lipoprotein, high total cholesterol, and high triglycerides. The HAART interferes with lipid-lowering drugs, especially statins, and both are metabolized by cytochrome P450 (except pravastatin).32 Until now, no clinical trials have been demonstrated to reduce CV events with lipid-lowering drugs in HIV-infected patients and consequently their indications are the same as in uninfected patients, based on calculation of overall CV risk. Small studies have demonstrated that atorvastatin at a dose of 40mg reduced the volume of noncalcified plaque, while rosuvastatin at a dose of 10mg decreased the carotid intima-media index. Thus, statins are the treatment of choice, being safe and effective (except simvastatin and lovastatin due to a higher risk of toxicity with PIs), and should be prescribed in all people with established vascular disease or with DM and/or a high risk of CV disease regardless of lipid levels. The drug of choice is atorvastatin, due to its efficacy profile, tolerability, experience with its use, and price. Currently, a clinical trial is underway (RETRIEVE: Randomized Trial to Prevent Vascular Events in HIV), which aims to randomize 6500 HIV patients, without CV disease, to statin vs placebo, in order to analyze in detail the usefulness of statins in primary prevention in HIV-infected patients. Ezetimibe can be used for HIV-infected patients who are intolerant to statins, or can be added to a statin when reductions in low-density lipoprotein are inadequate despite the maximum tolerated dose of statin. Fibrates–gemfibrozil and fenofibrate–can be used for the treatment of severe hypertriglyceridemia (> 500mg/dL) that is refractory to diet and in persons with a history of associated pancreatitis. The combination of statins and fibrates is not recommended because it systematically favors the toxicity of both drugs. In patients with multiple CV risk factors, we recommend starting HAART with drugs that have a good metabolic profile. In patients already treated with HAART who develop metabolic disorders or worsening CV risk, it is recommended to evaluate a change in the antiretroviral drugs, even before the initiation of lipid-lowering therapy, provided that their effectiveness is not compromised.
Hypertension has been reported to be present in more than 28% of HIV-infected patients, with a rate which rises as age increases in this population.33 The therapeutic management and goals are the same as in the general population. The threshold blood pressure is < 140/90mmHg and < 130/80mmHg is considered appropriate in diabetic patients. As for therapy, the drugs of choice seem to be angiotensin-converting enzyme inhibitors, due to their safety profile and metabolic benefits (especially in prediabetic and diabetic patients). Angiotensin II receptor antagonists can also be safely used (especially valsartan, candesartan and olmesartan). With calcium antagonists, dose adjustment and monitoring of possible adverse effects are required due to their possible interactions. Beta-blockers are safe, especially atenolol, whereas there is little information on the drug interactions of diuretics, especially with PIs, although they seem safe.
ACUTE MYOCARDIAL INFARCTIONPatients with HIV infection are at higher CV risk than the general population, with a 1.5- to 2-fold increased risk of AMI and at younger ages.34 In the DAD study, the frequency of AMI increased from 0.27 per 1000 people per year in persons aged between 25 and 30 years to 16.99 per 1000 people per year in persons older than 70 years.35 It has been proposed that immunologic changes in HIV patients are similar to those occurring with age (decreased CD4), suggesting that the virus causes premature aging of the immune system, a process known as immunosenescence. The AMI incidence rate was 0.43 cases per 1000 person-years among persons with a 5-year history of HIV infection or less, 0.86 cases per 1000 person-years in those with a 5- to 10-year history of HIV infection, 1.06 cases per 1000 person-years among those with a 10- to 15-year history, and 2.65 cases per 1000 person-years in those who had been living with HIV for more than 15 years. One possible explanation is exposure to antiretroviral drugs.36,37 The HOPS study showed an association between PI therapy and the development of AMI, which remained unchanged when adjusted for other CV risk factors.38 In addition, AMI risk significantly increased when CD4 counts fell below 100 cells/μl. After adjustment for age, exposure to antiretroviral drugs, CD4 count nadir and current CD4 levels, it was seen that a 10-year history of HIV infection increased the risk of AMI in the same way as aging 10 years, so a 40-year-old who was infected at the age of 30 years has an AMI risk similar to that of a 50-year-old without HIV infection. Thus, physicians should consider the timing of HIV infection as an independent CV risk factor and promote a healthy lifestyle from a CV standpoint (eg, weight control, heart-healthy diet, exercise, smoking cessation) among patients with long-standing HIV infection.
To compare the prognosis of HIV patients after an AMI with those not infected with the virus, an American team of researchers evaluated AMI-associated mortality in a group of patients between 1997 and 2006, using data from the Nationwide Inpatient Sample.39 HIV-infected patients were significantly younger (48 and 54 years, respectively; P < .001) and were less likely to undergo coronary angiography (48% vs 63%). After adjustment of the results for age, sex, ethnicity, comorbidities, type of hospital and the number of hospital procedures undergone by the participants, the risk of in-hospital death from AMI was 38% higher in the HIV-infected group (odds ratio = 1.38; 95% confidence interval, 1.01 to 1.87; P = 0.04). Therefore, during hospitalization due to AMI, mortality in people with HIV is higher than in the general population, which should be taken into account by physicians treating HIV patients hospitalized for this cause. Moreover, mortality is not only higher during the in-hospital phase, but also throughout follow-up, with higher rates of stent restenosis, as well as a greater number of readmissions for CV causes, as demonstrated in the French registry, whose results of a comparison of 608 HIV-infected patients with AMI vs 1216 uninfected patients were published in 2013.40
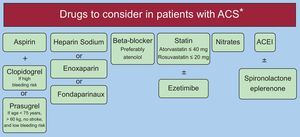
Therefore, because coronary atherosclerotic disease occurs earlier and has a worse prognosis in HIV patients, we must focus our efforts on more aggressive management of these patients, both from the point of view of interventionist and medical management. In this way, HIV-infected patients with AMI should undergo early coronary angiography and percutaneous revascularization, using the new-generation drug-eluting stents. As for antithrombotic therapy, dual antiplatelet therapy should be provided with clopidogrel or prasugrel (the latter should be used in patients with a low bleeding risk, age < 75 years, weight > 60kg, and no history of previous stroke). Ticagrelor has important interactions with PIs. These patients should be treated with beta-blockers (atenolol as first choice) and potent statins (atorvastatin 40mg or rosuvastatin 10-20mg), as well as with angiotensin-converting enzyme inhibitors and eplerenone, depending on whether or not there is an indication. Regarding antiangina medications, nitrate use is safe, but drugs such as ranolazine and ivabradine should not be indicated in patients receiving PIs (Figure 5).
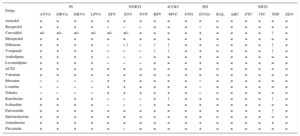
DRUG INTERACTIONSA significant number of patients with HIV infection are treated with several medications in addition to antiretroviral drugs. The interactions of antiretroviral drugs with each other or with other drugs may have a significant clinical impact. The most important interactions are usually pharmacokinetic (resulting in a change of concentration), especially those affecting metabolism. Table 4, Table 5, and Table 6 show the most frequent interactions of CV drugs with antiretroviral therapy using a color code: red indicates no recommendation on the drug combination, orange indicates that the drug is recommended with dose adjustment, yellow indicates the existence of a weak interaction not requiring dose adjustment, and green indicates no interaction. Before a CV therapy is prescribed to an HIV-infected patient, it is necessary to ensure that there are no relevant interactions with HAART, in order to select the treatment with the best effectiveness/safety profile.
Interaction of Antiretroviral Drugs With Antiplatelet and Anticoagulant Drugs
| Drugs | PI | NNRTI | iCCR5 | INI | NRTI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATV/r | DRV/c | DRV/r | LPV/r | EFV | ETV | NVP | RPV | MVC | DTG | EVG/c | RAL | ABC | FTC | 3TC | TDF | ZDV | |
| Aspirin | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | * | = |
| Clopidogrel | – | – | – | – | + | – | + | = | = | = | – | = | = | = | = | = | = |
| Prasugrel | – | – | – | – | = | = | = | = | = | = | – | = | = | = | = | = | = |
| Ticagrelor | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Heparin sodium | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Enoxaparin | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Fondaparinaux | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Acenocumarol | – | – | – | – | – | – | – | = | = | = | – | = | = | = | = | = | = |
| Warfarin | +/– | – | – | – | +/– | – | +/– | = | = | = | – | = | = | = | = | = | = |
| Dabigatran | + | + | + | + | = | = | = | + | = | = | + | = | = | = | = | = | = |
| Rivaroxaban | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Apixaban | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Edoxaban | + | + | + | + | = | = | = | = | = | = | + | = | = | = | = | = | = |
3TC, lamivudine; ABC, abacavir; ATV/r, atazanavir with ritonavir as a booster; DRV/c, darunavir with cobicistat as a booster; DRV/r, darunavir with ritonavir as a booster; DTG, dolutegravir; EFV, efavirenz; ETV, etravirine; EVG/c, elvitegravir with cobicistat as a booster; FTC, emtricitabine; iCCR5, inhibitor of cochemokine receptor 5; INI, integrase inhibitors; LPV/r, lopinavir with ritonavir as a booster; MVC, maraviroc; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NVP, nevirapine; PI: protease inhibitors; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir; ZDV, zidovudine.
Color code: red indicates no recommendation on the drug combination, orange indicates that the drug is recommended with dose adjustment, yellow indicates the existence of a weak interaction not requiring dose adjustment, and green indicates no interaction.
– Denotes a potential decrease of the drug considered in the first column.
+ Denotes a potential increase of the plasma concentration of the drug considered in the first column.
+/– Denotes a potential increase or decrease of the drug in the first column (not predictable).
= No significant interaction.
*Nefrotoxicity.
Interaction of Antiretroviral Drugs With Other Cardiovascular Drugs
| Drugs | PI | NNRTI | iCCR5 | INI | NRTI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATV/r | DRV/c | DRV/r | LPV/r | EFV | ETV | NVP | RPV | MVC | DTG | EVG/c | RAL | ABC | FTC | 3TC | TDF | ZDV | |
| Atenolol | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Bisoprolol | + | + | + | + | – | – | – | = | = | = | – | = | = | = | = | = | = |
| Carvedilol | +/– | +/– | +/– | +/– | +/– | +/– | = | = | = | = | + | = | = | = | = | ↑ | = |
| Metoprolol | + | + | + | + | = | = | = | = | = | = | + | = | = | = | = | = | = |
| Diltiazem | + | + | + | + | – | – ↑ | – | ↑ | ↑ | = | + | = | = | = | = | ↑ | = |
| Verapamil | + | + | + | + | – | – | – | ↑ | ↑ | = | + | = | = | = | = | ↑ | = |
| Amlodipine | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Lecarnidipine | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| ACEI | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Valsartan | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Irbesatan | – | – | – | – | + | + | = | = | = | = | – | = | = | = | = | = | = |
| Losartan | – | – | – | – | + | + | = | = | = | = | – | = | = | = | = | = | = |
| Nitrates | – | – | – | – | + | + | + | = | + | = | – | = | = | = | = | = | = |
| Ranolazine | + | + | + | + | – | – | – | + | ↑ | = | + | = | = | = | = | ↑ | = |
| Ivabradine | + | + | + | + | – | – | – | + | = | = | + | = | = | = | = | = | = |
| Furosemide | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | ↑ | = |
| Spironolactone | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Amiodarone | + | + | + | + | = | = | = | = | = | = | + | = | = | = | = | + | = |
| Flecainide | + | + | + | + | = | – | = | = | = | = | + | = | = | = | = | = | = |
3TC, lamivudine; ABC, abacavir; ACEI, angiotensin-converting enzyme inhibitors; ATV/r, atazanavir with ritonavir as a booster; DRV/c, darunavir with cobicistat as a booster; DRV/r, darunavir with ritonavir as a booster; DTG, dolutegravir; EFV, efavirenz; ETV, etravirine; EVG/c, elvitegravir with cobicistat as a booster; FTC, emtricitabine; iCCR5, inhibitor of co-chemokine receptor 5; INI, integrase inhibitors; LPV/r, lopinavir with ritonavir as a booster; MVC, maraviroc; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NVP, nevirapine; PI: protease inhibitors; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir; ZDV, zidovudine.
Color code: red indicates no recommendation on the drug combination, orange indicates that the drug is recommended with dose adjustment, yellow indicates the existence of a weak interaction not requiring dose adjustment, and green indicates no interaction.
– Denotes a potential decrease of the drug considered in the first column.
+ Denotes a potential increase of the drug considered in the first column.
+/– Denotes a potential increase or decrease of the drug in the first column (not predictable).
↑ Denotes potential increased plasma levels of antiretroviral drug.
= No significant interaction.
Interaction of Antiretroviral Drugs With Lipid-lowering Drugs
| Drugs | PI | NNRTI | iCCR5 | INI | NRTI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATV/r | DRV/c | DRV/r | LPV/r | EFV | ETV | NVP | RPV | MVC | DTG | EVG/c | RAL | ABC | FTC | 3TC | TDF | ZDV | |
| Atorvastatin | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Fluvastatin | = | = | = | = | + | + | = | = | = | = | + | = | = | = | = | = | = |
| Lovasatin | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Pitavastatin | + | + | + | + | = | = | = | = | = | = | + | = | = | = | = | = | = |
| Pravastatin | = | + | + | = | – | = | = | = | = | = | + | = | = | = | = | = | = |
| Rosuvastatin | + | + | + | + | = | = | = | = | = | = | + | = | = | = | = | = | = |
| Simvastatin | + | + | + | + | – | – | – | = | = | = | + | = | = | = | = | = | = |
| Fenofibrate | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
| Gemfibrozil | – | – | – | – | = | = | = | = | = | ↑ | = | ↑ | = | = | = | = | = |
| Ezetimibe | + | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = | = |
3TC, lamivudine; ABC, abacavir; ATV/r, atazanavir with ritonavir as a booster; DRV/c, darunavir with cobicistat as a booster; DRV/r, darunavir with ritonavir as a booster; DTG, dolutegravir; EFV, efavirenz; ETV, etravirine; EVG/c, elvitegravir with cobicistat as a booster; FTC, emtricitabine; iCCR5, inhibitor of cochemokine receptor 5; INI, integrase inhibitors; LPV/r, lopinavir with ritonavir as a booster; MVC, maraviroc; NNRTI, nonnucleoside reverse transcriptase inhibitors; NRTI, nucleoside reverse transcriptase inhibitors; NVP, nevirapine; PI: protease inhibitors; RAL, raltegravir; RPV, rilpivirine; TDF, tenofovir; ZDV, zidovudine.
Color code: red indicates no recommendation on the drug combination, orange indicates that the drug is recommended with dose adjustment, yellow indicates the existence of a weak interaction not requiring dose adjustment, and green indicates no interaction.
– Denotes a potential decrease of the drug considered in the first column.
+ Denotes a potential increase of the drug considered in the first column.
↑ Denotes potential increased plasma levels of antiretroviral drug.
= No significant interaction.
Another aspect to consider is the high prevalence of HIV and hepatitis C virus coinfection, because both viruses share transmission routes. Therefore, given that treatments for both entities are frequently coadministered in these patients, and that new therapies for hepatitis C have recently entered the market, it is essential to take into account the possible interactions between HAART and these new drugs.41
CONCLUSIONSAt present, the most common CV disease associated with HIV in developed countries with access to antiretroviral therapy is ischemic heart disease. HIV patients have a poorer cardiovascular profile, secondary both to infection itself and to antiretroviral treatment, and prognosis is also less favorable than that in uninfected patients. In addition, when treating HIV patients with ischemic heart disease, physicians must be familiar with the drug interactions between antithrombotic therapy and drugs used to control cardiovascular risk.
CONFLICTS OF INTERESTNone declared.