Keywords
INTRODUCTION
In 1980, the first reports of surgery for patients with hypoplastic left heart syndrome were published,1 although in-hospital mortality was high initially.2 The alternative of neonatal transplantation showed early promise3 but the limitations of finding donors of low body weight quickly became apparent. In recent years, progress in surgical techniques4 and the improved outcomes in stage I of the Norwood procedure5 have improved the possibility of survival in neonates with hypoplastic left heart syndrome. However, patients who survive neonatal surgery may develop obstruction in the aortic arch, distortion of the pulmonary arteries,6-8 right ventricular dysfunction, or tricuspid regurgitation, which can prevent completion of the Fontan procedure and lead to higher interstage mortality than observed in other forms of univentricular circulation.9 Early detection of these complications by imaging techniques (ultrasound, magnetic resonance imaging [MRI], multislice computed tomography [CT]), and treatment by catheterization techniques are essential for improving long-term survival and quality of life of these patients. Thus, follow-up of these children from stage I onwards remains a challenge for the medical and surgical personnel involved in treatment.
METHODS
From December 1992 to December 2006, 30 patients with diagnosis of hypoplastic left heart syndrome underwent the Norwood procedure: 24 by means of the classic Norwood procedure and 6 by the modified Norwood procedure (Sano technique). The survival rate was 56% (17/30). Between February 1993 and December 2006, 25 interventional procedures were performed prior to a Glenn or Fontan procedure in 14 of the 17 patients who survived neonatal surgery. We review the medical histories, surgery reports, and angiographic and hemodynamic findings in these 14 patients. The neonatal diagnoses were mitral-aortic atresia with ascending aorta diameters between 1.5 and 5 mm (n=11), Shone syndrome (n=1), mitral atresia with ventricular shunting and aortic arch hypoplasia (n=1), and single ventricle with arch hypoplasia (n=1). Ten patients underwent an operation following the classic Norwood procedure (modified right Blalock-Taussig shunt) and 4 following the modified Sano technique (right ventricle-pulmonary artery conduit). The age at which interventional catheterization was done ranged from 2 months to 11.9 years (mean, 2.1 years) and the weight ranged from 3.3 to 31 kg (mean [SD], 9.7 [6.7] kg). The follow-up time after neonatal surgery was 3.7 (4.3) years (range, 6 months-13.5 years). Catheterization was done under general anesthetic and orotracheal intubation--with administration of cefazolin as a prophylactic antibiotic--by percutaneous femoral artery and vein puncture or by puncture of the jugular vein. In the hemodynamic study after stage I, particular attention was paid to ruling out obstruction of the neoaorta (peak gradient, >15 mm Hg), atrial gradient, right ventricular function, state of the Blalock-Taussig shunt or the right ventricle-pulmonary artery conduit, pulmonary pressures and resistances, and anatomy of the pulmonary tree. After stage II (Glenn procedure), we also studied stenosis of the anastomosis between the superior vena cava and the right pulmonary artery, the possibility of veno-venous connections between the superior vena cava and the inferior vena cava, the anatomy of the inferior vena cava and suprahepatic veins, and the patency of access to the femoral arteries and femoral and jugular veins. In the follow-up after the interventional procedures, ultrasound was used in all patients, MRI in 8, multislice CT in 1, and catheterization in 5.
RESULTS
Recoarctation
In 8 (57%) of the 14 patients, significant obstruction was observed in the neoaortic arch (peak right ventricular-descending aorta gradient ≥15 mm Hg, with angiographic imagining indicating a reduction in diameter of >50%). We did not find any significant differences in the incidence of recoarctation between patients in whom heterologous tissue had been used for aortic arch reconstruction (5/9) and those in whom such tissue was not used (3/5). The time elapsed between performing stage I of the Norwood procedure and recoarctation was 9.5 months (4 months-2.5 years). Nine angioplasties were performed in 7 patients (in a patient, who was referred for surgery, angioplasty was ruled out due to hemodynamic instability) using the femoral artery (retrograde direction) in 7 and the femoral venous approach (retrograde direction) in 2 (due to femoral artery stenosis). Initially effective angioplasty was achieved in 6 out of 7 patients (85%) (Figure 1), but 2 presented new recoarctation during follow-up and were submitted to a second angioplasty procedure, which was effective in 1 of them. The other patient was referred for surgery. The characteristics of the patients undergoing recoarctation angioplasty, as well as the angiographic and hemodynamic data, are summarized in Table 1.
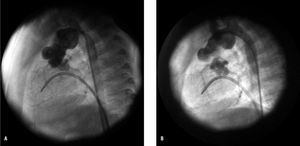
Figure 1. A: recoarctation in a 4-month-old patient weighing 3.3 kg (surgical technique: arch reconstruction without heterologous material), with maximum gradient of 35 mm Hg and lesion diameter of 2.8 mm. B: after angioplasty with 7-mm Tyshak mini balloon (Numed®), gradient of 0 mm Hg and stenosis diameter of 5 mm.
Pulmonary Artery Angioplasty
With regard to pulmonary artery involvement, 10 angioplasties were done in 7 patients (3 cases showed early restenosis after an initially ineffective angioplasty). Of the 10 children who underwent the classic Norwood procedure (Blalock-Taussig shunt to right pulmonary artery), we found detachment of the left pulmonary artery in 2 patients and critical stenosis at its root in a third. The patient with critical stenosis underwent balloon angioplasty through the surgical shunt, with a good outcome, but after a few months, the left pulmonary artery detachment progressed, and finally, a fenestrated Fontan procedure to the right lung was done; currently, the patient has a saturation of 85% and is functional grade I. A further 3 patients who underwent the classic Norwood procedure required, after stage II and before performing the Fontan procedure, stent angioplasty of the pulmonary arteries, which was done by the jugular vein approach (Glenn procedure) with 26-mm EV3 (n=1), Numed CP (n=1, Figure 2), and Palmaz P188 (n=1) stents. In 1 of these patients, the stent was redilated 1 year after deployment and the Fontan procedure was successfully completed in all 3 patients.
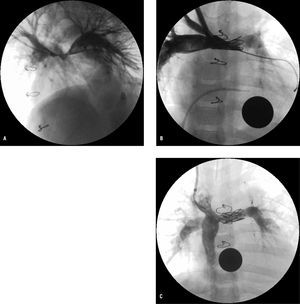
Figure 2. A: angiography (left anterior oblique view) after classic Norwood and Glenn procedure in 3-year-old patient who developed severe hypoplasia where the pulmonary arteries meet. B: anteroposterior angiography after balloon angioplasty and subsequent deployment of 22-mm CP stent (Numed). C: inferior vena cava angiography after completing Fontan procedure 6 months after stent redilation.
Of the 4 patients who underwent a Sano procedure, 3 required pulmonary artery angioplasty, which was done in 2 cases with a balloon catheter (due to the low weight of the patients and the small size of the pulmonary arteries) via the conduit or the Glenn shunt. In the other patient, in whom a double Glenn procedure was done due to persistence of the left superior vena cava, a 22-mm Numed CP stent was deployed using an 8-mm balloon-in-balloon catheter in the segment of the pulmonary arteries between the 2 venae cavae, which had been steadily becoming hypoplastic (Figure 3). The angiographic and hemodynamic data and information on the technique used in the pulmonary artery angioplasties are presented in Table 2.
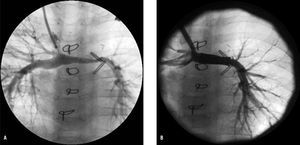
Figure 3. A: angiograph of superior vena cava in a patient after the Norwood, Sano, and Glenn procedure, with double superior vena cava, who developed left pulmonary artery hypoplasia (minimum caliber of 2.2 mm). B: angiography after deployment of 22-mm CP stent on an 8-mm balloon-in-balloon catheter: the left pulmonary artery now measures 6.4 mm in diameter.
Embolizations
In a patient with the Glenn procedure, embolization (with coils) of the collateral veins from the right superior vena cava to the inferior vena cava territory was required. This improved saturation from 75% to 84%. Another girl with the Glenn procedure developed repatency of the left superior vena cava at the coronary sinus (embolization with the 6/4 Amplatzer duct occluder by anterograde venous approach along the femoral vena cava; Figure 4); in another boy who had undergone the Glenn procedure and left Blalock-Taussig shunt to favor development of his left pulmonary artery, which showed extensive hypoplasia, embolization was done with the 6/4 Amplatzer duct occluder on completing the Fontan procedure.
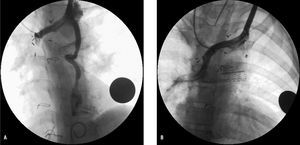
Figure 4. A: angiograph of the innominate vein in a patient after the Glenn procedure, showing repatency of the left superior vena cava at the coronary sinus. B: angiograph of the innominate vein after embolization with the 6/4 Amplatz duct occluder, introduced via the femoral vein with a venovenous wire loop.
Angioplasty With Balloon Catheter in the Glenn Procedure
This procedure was done in 2 patients and was effective in both (the gradient and the superior vena cava syndrome disappeared), with no signs of restenosis during follow-up.
Fibrinolysis
A female patient aged 16 months old and weighing 8 kg, with a double superior vena cava, also presented thrombosis of the left superior vena cava and left pulmonary artery on the ninth day after the Glenn procedure; to avoid a repeat surgical intervention, fibrinolysis was done with urokinase infused through a 5 Fr catheter in the left superior vena cava, with no bleeding complications. Other findings in the angiographic studies of these patients, and for whom no interventional techniques were practiced, were stenosis of the supraaortic trunk in 6 patients, stenosis of the femoral arteries (n=2), and femoral vein thrombosis (n=5) or subclavian vena cava thrombosis (n=1).
The following were reported as complications of interventional catheterization: arterial ischemia in 2 patients, treated in 1 case by heparin perfusion for 24 hours and in the other by fibrinolysis with tissue plasminogen activator (r-TPA); cardiac arrest or bradycardia that required resuscitation (n=4); and transient atrioventricular block (n=1).
DISCUSSION
Although presentation of the results of surgery in stage I of the Norwood procedure is not the focus of this study, the survival of 56% reported is a reflection of how we are learning and the limited number of patients who underwent the operation in this period. This figure coincides with the results published recently by another Spanish medical-surgical team.10
The appearance of obstructive gradients in the aortic arch is one of the most common complications described after the Norwood procedure and detection is not always easy with ultrasound techniques.11 The development of recoarctation has been associated in these patients with right ventricular dysfunction, tricuspid regurgitation, decreased systemic flow, and an increase in the interstage mortality.8,12 Therefore, angioplasty is indicated in these patients for gradients less than those employed for simple recoarctation (not associated with complex heart disease), provided the angiographic image is indicative of significant stenosis (decrease in aortic diameter >40%-50%12). Given that stenosis of the supraaortic trunk is common, a small or nonexistent pressure gradient as determined by noninvasive techniques did not rule out the indication of angioplasty if the hemodynamic gradient and the angiographic image pointed to significant stenosis. The published incidence of recoarctation ranges from 9.2% to 45%13-15; these differences can be explained by the different surgical techniques used in arch reconstruction,16 the experience of the surgical teams (lower incidence of recoarctation in larger series), different definitions of recoarctation used (in some studies, hemodynamic gradients are considered as >25, 17, or 20 mm Hg,13 whereas in others they are considered as >10 or 15 mm Hg,18 and some even use angiographic criteria16), and the different follow-up times. The high incidence of recoarctation observed in our series is explained by the fact that we included patients with gradients ≥15 mm Hg, had a longer follow-up than some series, and, of course, by the fact that ours was a relatively small series that included our initial experience with stage I of the Norwood procedure. Our findings with regard to balloon angioplasty in recoarctation, with an initial success of 85%, are similar to those published by other groups (82%-100%),12,13,18,19 as is the incidence of new recoarctation after the first angioplasty (28% of our series and between 20%13 and 44% in the literature19). The anatomic peculiarities of the neoaorta, as well as the young age at which these patients presented with recoarctation, was responsible in some cases for the need to use relatively large balloons compared to the weight of the children, something which led to acute arterial complications (2/7 patients) and long-term stenosis of the femoral arteries. In recent years, the introduction of catheters with a smaller cross-section (Tyshak mini, Numed) has led to a decrease in the incidence of this type of complication. Anterograde approach, from the femoral vein, allows the use of introducers with a larger cross-section, but stabilization of the balloon during angioplasty is more difficult and there is a risk of lesion to the tricuspid and pulmonary valves (which are now the systemic valves, and the competency of which is essential for the Fontan physiology). In addition, in line with what has been published,12,18,20,21 percutaneous angioplasty in these patients is associated more often with hemodynamic instability and even with the need for cardiopulmonary resuscitation during the procedure than in other recoarctation patients. In our series, this complication was observed in 2 patients; in 1 the angioplasty could be completed while the other was referred for surgery.
In terms of pulmonary artery involvement, the modified Sano technique (shunt from the right ventricle) prevented the problem of detachment or hypodevelopment of the left pulmonary artery in our patients but it did not prevent stenosis of the pulmonary arteries where the conduit was inserted.22 The incidence of significant stenosis of pulmonary branches after Norwood surgery (before the Glenn or Fontan procedure or even after this procedure) reported in the literature ranges between 40% and 62%.23,24 In our series, we observed in particular a tendency towards presenting severe stenosis and/or hypodevelopment where the 2 pulmonary arteries meet and where residual ductal tissue may remain. This second possibility is difficult to access for the surgeon as it is in a posterior position to the usually dilated ascending neoaorta. Another factor potentially involved in the hypodevelopment of this region is the presence of double superior vena cava, as after the Glenn procedure the flow to each superior vena cava is directed towards the ipsilateral pulmonary artery; 2 of the 3 patients who needed stenting in this region had a double superior vena cava. Another of the peculiarities of angioplasty of the pulmonary arteries in these patients after stages I or II is that they had a high incidence of restenosis (possibly due to presence of residual ductal tissue). These patients also tend to have a low body weight and small pulmonary arteries that preclude the possibility of placing breakable stents with the possibility of redilation. For follow-up of these stenoses after angioplasty, MRI or CT scans are essential. Deployment of smaller stents (coronary or peripheral) that require smaller introducers could be an option, but this would limit the development of the artery and would end up producing stenosis with the somatic growth of the patient. In our patients, we tried using balloon angioplasty in the smallest patients (although redilation due to short-term or medium-term restenosis was required), and delaying stent placement to allow stents with the possibility of redilation to be deployed (Palmaz, Ev3, or Numed CP) with 10-11 Fr introducers, with approach without complications through the jugular vein in children up to 10 kg in body weight. Perhaps, in the future, use of new pediatric stents, such as reabsorbable ones25 (Biotronik) or small cross-section breakable stents with the possibility of redilation (growth stent),26 still in clinical development, could facilitate and improve treatment of these lesions. On the other hand, stenting where the pulmonary arteries meet could affect the surgical technique used in stage III: in 2 of our patients with stenting at this position, the extracardiac conduit from the lower vena cava had to be shunted to the right pulmonary artery, opposite the Glenn shunt (Figure 2B), with no complications reported during the postoperative period.
The development of collateral venous vessels and recanalization of embryologic vessels from the territory of the superior vena cava are complications described after Glenn surgery,27 and are more common in patients with higher transpulmonary gradient (pulmonary arteriolar resistance, that is, difference between mean pulmonary pressure and mean pressure of the left atrium).28 This may require embolization due to significant hypoxemia, as occurred in 2 of our patients.
The role of interventional catheterization is crucial in the follow-up of patients who, after stage I of the Norwood procedure (neonatal surgery with complex reconstruction of the aortic arch and sutures in the pulmonary tree), have to achieve optimal conditions for the Fontan procedure. The improvement in surgical techniques and the increased experience of the personnel probably decrease the incidence in the short term of recoarctation or distortion of the pulmonary branches, but we should not forget that patients undergo substantial somatic growth before and after completing stage III.
In the future, new hybrid techniques for stage I (banding of pulmonary arteries plus stenting of the ductus, angioplasty of coarctation, and extension of the atrial septum by percutaneous interventions),29 although still under clinical development, might strengthen the role of interventional catheterization in the management of hypoplastic left heart syndrome.
CONCLUSIONS
After Norwood surgery, an early angiographic and hemodynamic assessment should be performed to detect and treat stenoses of the pulmonary arteries and/or the aortic arch. Although angioplasty of the recoarctation may be effective, restenosis is common. The Sano technique prevents detachment of the left pulmonary artery, but stenoses may also appear in the pulmonary arteries. After stage II, it is necessary to carry out studies to determine whether venovenous collateral vessels are present as these often require embolization. Therapeutic catheterization in these patients is associated with a higher incidence of major complications than in other patient groups.
ABBREVIATIONS
AV: atrioventricular
BIB: "balloon-in-balloon"
CT: computed tomography
MRI: magnetic resonance imaging
Correspondence: Dra. M.J. del Cerro Marín.
Servicio de Cardiología Pediátrica. Hospital Infantil La Paz.
P.o de la Castellana, 261. 28046 Madrid. España.
E-mail: mcerro.hulp@salud.madrid.org
Manuscript received May 14, 2007.
Accepted for publication October 10, 2007.