Heart failure patients with nonvalvular atrial fibrillation (NVAF) on treatment with vitamin K antagonists (VKA) often have suboptimal international normalized ratio (INR) values. Our aim was to evaluate the association between INR values at admission due to acute heart failure and mortality risk during follow-up.
MethodsIn this observational study, we retrospectively assessed INR on admission in 1137 consecutive patients with acute heart failure and NVAF who were receiving VKA treatment. INR was categorized into optimal values (INR = 2-3, n = 210), subtherapeutic (INR < 2, n = 660), and supratherapeutic (INR > 3, n = 267). Because INR did not meet the proportional hazards assumption for mortality, restricted mean survival time differences were used to evaluate the association among INR categories and the risk of all-cause mortality.
ResultsDuring a median [interquartile range] follow-up of 2.15 years [0.71-4.29], 495 (43.5%) patients died. On multivariable analysis, both patients with subtherapeutic and supratherapeutic INR showed higher risks of all-cause mortality, as evidenced by their restricted mean survival time differences at 5 years’ follow-up: –0.50; 95%CI, –0.77 to –0.23 years; P < .001; and –0.40; 95%CI, –0.70 to –0.11 years; P = .007, respectively, compared with INR 2-3.
ConclusionsIn acute heart failure patients on treatment with VKA for NVAF, INR values out of normal range at admission were independently associated with a higher long-term mortality risk.
Keywords
Atrial fibrillation and heart failure are closely associated, both clinically and pathophysiologically.1 Atrial fibrillation is the most common arrhythmia in patients with heart failure and is associated with an increased risk of thromboembolic events, worse functional class, and worse prognosis.2 Because of the risk of stroke or systemic embolism, the vast majority of heart failure patients with nonvalvular atrial fibrillation (NVAF) need long-term oral anticoagulant therapy.3 Nevertheless, and despite recent evidence in favor of direct oral anticoagulants,4,5 a large proportion of patients with heart failure and NVAF in Spain are treated with vitamin K antagonists (VKAs). Management of oral anticoagulant therapy in the setting of heart failure, however, is difficult because of multiple drug interactions, concomitant liver or kidney failure, and greater international normalized ratio (INR) instability.6,7 This is particularly true during acute decompensated heart failure, as heart failure patients often have INR values outside the optimal therapeutic range.8
INR was also recently associated with mortality risk in patients with acute heart failure who were not receiving anticoagulants.9 Pathophysiologic mechanisms that could explain the importance of INR in heart failure include tissue hypoxia, liver dysfunction, and the degree of neurohormonal activation. The prognostic value of INR in patients on VKA therapy, however, is unknown. The aim of this study was to assess the association between INR and long-term mortality risk in patients with acute heart failure and NVAF being treated with a VKA.
METHODSStudy Group and ProtocolWe studied a consecutive cohort of 2604 patients with a primary diagnosis of acute heart failure admitted to the cardiology ward of a tertiary care university hospital between January 2004 and December 2016. Acute heart failure was defined according to clinical practice guidelines.10 Patients with acute decompensated heart failure resulting from new-onset or chronic heart failure were included. Of the 2604 patients in the registry, 1467 were excluded because a) they did not have a diagnosis of atrial fibrillation (n = 1262), b) they did not have a diagnosis of valvular atrial fibrillation according to the recommendations of the European Cardiology Society3 (n = 157), or c) they were being treated with an anticoagulant other than a VKA (n = 48). The final sample thus consisted of 1137 patients.
A wide range of variables, including clinical, physical examination, biochemical, electrocardiographic, and echocardiographic variables, together with details of concomitant treatments, were recorded during the initial hospital stay. Treatment was individualized at the discretion of the attending cardiologist and in accordance with clinical practice guidelines applicable over the study period.
The study was performed in accordance with the principles set forth in the Declaration of Helsinki and was approved by ethics committee of the hospital.
INR ValuesINR was measured during the first medical contact in the emergency department. A value of 2 to 3 was considered to be within the optimal therapeutic range. Patients were classified into 3 categories depending on their INR value: a) a therapeutic category (INR 2-3), b) a subtherapeutic category (INR < 2), and c) a supratherapeutic category (INR > 3).
Follow-up and Assignment of EventsSurvival after hospital discharge was checked by reviewing the patients’ electronic medical records. The researchers responsible for assigning the events did not have access to the patients’ INR data. The primary endpoint was the association between INR and all-cause mortality during follow-up. The secondary endpoint was the association between INR and cause-specific mortality, categorized as a) cardiovascular vs noncardiovascular death) and b) death due to heart failure vs death due a cause other than heart failure. Cardiovascular death was defined using the criteria recommended by the American Heart Association and included sudden death attributable to heart failure, acute myocardial infarction, stroke, vascular bleeding, or peripheral artery disease, and death due to an unknown cause. In all other cases, the cause of death was considered to be noncardiovascular.11
Statistical AnalysisContinuous variables are expressed as means ± standard deviation or, when nonnormally distributed, as medians and interquartile range. Discrete variables are expressed as percentages. The baseline characteristics of patients in the INR categories were compared using analysis of variance. The Kruskal-Wallis test was used for nonparametric variables.
The proportional hazards assumption was tested for all the study variables in the 3 INR categories using scaled Schoenfeld residuals and log-log curves. A different method of analysis was used depending on whether the assumption of proportional hazards was met or not. In the first case, a Royston-Parmar model was used and in the second case, differences in restricted mean survival time (RMST) were calculated.12 RMST is an alternative to the hazard ratio for situations in which the assumption of proportional hazards does not hold. RMST differences represent the years of life lost associated with a given study variable.12,13 The variables for which IRN did not meet the proportional hazards assumption were all-cause mortality, cardiovascular death, and death due to heart failure. The assumption was met for noncardiovascular death and death due to a cause other than heart failure. The maximum follow-up time in the RMST analysis was set at 5 years. Results are expressed as the difference in number of years up to the event.
All the variables in Table 1 were evaluated for their prognostic value. Backward elimination was used to simplify the final multivariate model. The optimal polynomial for the continuous variables was calculated during stepwise selection to ensure linearity with the event. Cumulative incidence curves were generated to analyze cause-specific mortality and differences were evaluated using the Gray test.14 RMST adapted to competing risks was used in the multivariate analysis of cause-specific mortality.
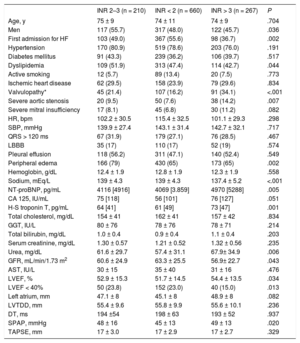
Patients’ Baseline Characteristics by INR Category
| INR 2–3 (n = 210) | INR < 2 (n = 660) | INR > 3 (n = 267) | P | |
|---|---|---|---|---|
| Age, y | 75 ± 9 | 74 ± 11 | 74 ± 9 | .704 |
| Men | 117 (55.7) | 317 (48.0) | 122 (45.7) | .036 |
| First admission for HF | 103 (49.0) | 367 (55.6) | 98 (36.7) | .002 |
| Hypertension | 170 (80.9) | 519 (78.6) | 203 (76.0) | .191 |
| Diabetes mellitus | 91 (43.3) | 239 (36.2) | 106 (39.7) | .517 |
| Dyslipidemia | 109 (51.9) | 313 (47.4) | 114 (42.7) | .044 |
| Active smoking | 12 (5.7) | 89 (13.4) | 20 (7.5) | .773 |
| Ischemic heart disease | 62 (29.5) | 158 (23.9) | 79 (29.6) | .834 |
| Valvulopathy* | 45 (21.4) | 107 (16.2) | 91 (34.1) | <.001 |
| Severe aortic stenosis | 20 (9.5) | 50 (7.6) | 38 (14.2) | .007 |
| Severe mitral insufficiency | 17 (8.1) | 45 (6.8) | 30 (11.2) | .082 |
| HR, bpm | 102.2 ± 30.5 | 115.4 ± 32.5 | 101.1 ± 29.3 | .298 |
| SBP, mmHg | 139.9 ± 27.4 | 143.1 ± 31.4 | 142.7 ± 32.1 | .717 |
| QRS > 120 ms | 67 (31.9) | 179 (27.1) | 76 (28.5) | .467 |
| LBBB | 35 (17) | 110 (17) | 52 (19) | .574 |
| Pleural effusion | 118 (56.2) | 311 (47.1) | 140 (52.4) | .549 |
| Peripheral edema | 166 (79) | 430 (65) | 173 (65) | .002 |
| Hemoglobin, g/dL | 12.4 ± 1.9 | 12.8 ± 1.9 | 12.3 ± 1.9 | .558 |
| Sodium, mEq/L | 139 ± 4.3 | 139 ± 4.3 | 137.4 ± 5.2 | <.001 |
| NT-proBNP, pg/mL | 4116 [4916] | 4069 [3.859] | 4970 [5288] | .005 |
| CA 125, IU/mL | 75 [118] | 56 [101] | 76 [127] | .051 |
| H-S troponin T, pg/mL | 64 [41] | 61 [49] | 73 [47] | .001 |
| Total cholesterol, mg/dL | 154 ± 41 | 162 ± 41 | 157 ± 42 | .834 |
| GGT, IU/L | 80 ± 76 | 78 ± 76 | 78 ± 71 | .214 |
| Total bilirubin, mg/dL | 1.0 ± 0.4 | 0.9 ± 0.4 | 1.1 ± 0.4 | .203 |
| Serum creatinine, mg/dL | 1.30 ± 0.57 | 1.21 ± 0.52 | 1.32 ± 0.56 | .235 |
| Urea, mg/dL | 61.6 ± 29.7 | 57.4 ± 31.1 | 67.9± 34.9 | .006 |
| GFR, mL/min/1.73 m2 | 60.6 ± 24.9 | 63.3 ± 25.5 | 56.9± 22.7 | .043 |
| AST, IU/L | 30 ± 15 | 35 ± 40 | 31 ± 16 | .476 |
| LVEF, % | 52.9 ± 15.3 | 51.7 ± 14.5 | 54.4 ± 13.5 | .034 |
| LVEF < 40% | 50 (23.8) | 152 (23.0) | 40 (15.0) | .013 |
| Left atrium, mm | 47.1 ± 8 | 45.1 ± 8 | 48.9 ± 8 | .082 |
| LVTDD, mm | 55.4 ± 9.6 | 55.8 ± 9.9 | 55.6 ± 10.1 | .236 |
| DT, ms | 194 ±54 | 198 ± 63 | 193 ± 52 | .937 |
| SPAP, mmHg | 48 ± 16 | 45 ± 13 | 49 ± 13 | .020 |
| TAPSE, mm | 17 ± 3.0 | 17 ± 2.9 | 17 ± 2.7 | .329 |
AST, aspartate aminotransferase; CA 125, carbohydrate antigen 125; DT, deceleration time; GFR, glomerular filtration rate; GGT, gamma-glutamyl transferase; HF, heart failure; HR, heart rate; H-S, high-sensitivity; INR, international normalized ratio; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NT-ProBNP, N-terminal probrain natriuretic peptide; LVTDD, left ventricular telediastolic diameter; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure; TAPSE, tricuspid anular plane systolic excursion.
Values are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
A 2-tailed P value of less than .05 was considered significant for all analyses. The statistical analyses were performed by MedStats Consulting (Reading, Pennsylvania, United States) using STATA 15.1 (StataCorp LP; College Station, Texas, United States).
RESULTSBaseline Patient CharacteristicsThe mean ± standard deviation age of the patients was 74 ± 10 years; 581 patients (51.1%) were women and 568 (50.2%) had already been hospitalized for heart failure. Most of the patients (n = 927, 81%) had an INR outside the therapeutic range. There were 210 patients (18.5%) in the therapeutic category (INR, 2-3), 660 (58.0%) in the subtherapeutic category (INR < 2), and 267 (23.5%) in the supratherapeutic category (INR > 3).
The patients’ baseline characteristics are shown by INR group in Table 1. INR values outside the therapeutic range, in particular, subtherapeutic values, were associated with various clinical, biochemical, and echocardiographic variables that are typically associated with poor prognosis in heart failure. Notably, there were no differences between the 3 groups for biochemical liver function parameters. Supratherapeutic INR values were more common in patients with preserved left ventricular ejection fraction than with reduced left ventricular ejection fraction (Table 1).
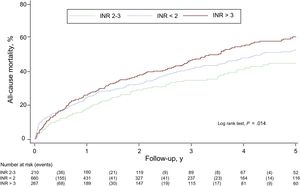
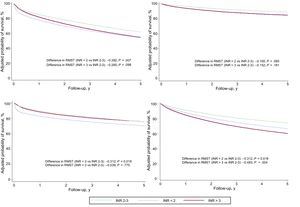
INR and All-Cause MortalityOver a median follow-up period of 2.15 (0.71-4.29) years, 495 patients (43.5%) died. Crude mortality rates were higher in patients with INR values outside the therapeutic range (Table 2). According to the Kaplan-Meier survival analysis, thus, the risk of all-cause mortality was higher in patients an INR value lower than 2 and in particular in those with an INR value above 3 (log rank test, P = .014) (Figure 1).
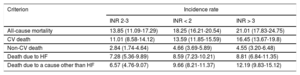
Crude Incidence Rates (per 100 Person-Years) and 95% Confidence Intervals (CIs) for All-Cause and Cause-Specific Mortality by INR Category
| Criterion | Incidence rate | ||
|---|---|---|---|
| INR 2-3 | INR < 2 | INR > 3 | |
| All-cause mortality | 13.85 (11.09-17.29) | 18.25 (16.21-20.54) | 21.01 (17.83-24.75) |
| CV death | 11.01 (8.58-14.12) | 13.59 (11.85-15.59) | 16.45 (13.67-19.8) |
| Non-CV death | 2.84 (1.74-4.64) | 4.66 (3.69-5.89) | 4.55 (3.20-6.48) |
| Death due to HF | 7.28 (5.36-9.89) | 8.59 (7.23-10.21) | 8.81 (6.84-11.35) |
| Death due to a cause other than HF | 6.57 (4.76-9.07) | 9.66 (8.21-11.37) | 12.19 (9.83-15.12) |
CV, cardiovascular; HF, heart failure; INR, international normalized ratio.
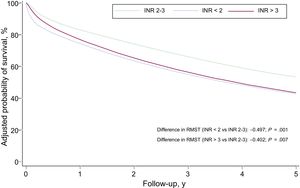
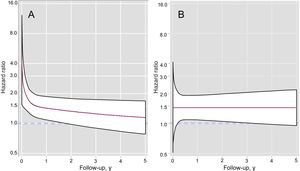
The multivariate analysis of RMST differences confirmed an independent association between INR on admission to hospital and an increased risk of all-cause mortality: RMSTINR<2 = –0.50 years (95% confidence interval [95%CI], –0.77 to –0.23) (P < .001) and RMSTINR>3 = –0.40 years (95%CI, –0.70 to –0.11) (P = .007) (Table 3). The Harrell C statistic was 0.771. The survival curves adjusted for all-cause mortality are shown by INR category in Figure 2. These curves were estimated from the multivariate RMST model and include the interaction between INR and time. On comparison of patients in the subtherapeutic and therapeutic categories, the strength of the association between subtherapeutic values and risk of all-cause mortality decreased over time and lost its significance in the second year. The association in the supratherapeutic group, by contrast, remained relatively constant over time but was only significant from the third month up to the third year (Figure 3). The modeling of time-dependent hazard ratios is presented in Table 1 of the supplementary material. When INR was established as a continuous variable in the multivariate model, both subtherapeutic and supratherapeutic INR values were found to be associated with an increased risk of all-cause mortality (P < .001) (Figure of the supplementary material).
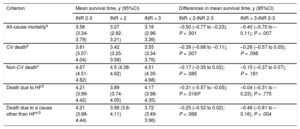
Risk Estimates (Differences in Mean Survival Times) in the Different Analyses of Primary and Secondary Endpoints in Subtherapeutic (INR < 2) and Supratherapeutic (INR > 3) Categories Compared With INR 2–3
| Criterion | Mean survival time, y (95%CI) | Differences in mean survival time, y (95%CI) | |||
|---|---|---|---|---|---|
| INR 2-3 | INR < 2 | INR > 3 | INR < 2-INR 2-3 | INR > 3-INR 2-3 | |
| All-cause mortalitya | 3.56 (3.34-3.79) | 3.07 (2.92-3.21) | 3.16 (2.96-3.36) | –0.50 (–0.77 to –0.23); P < .001 | –0.40 (–0.70 to –0.11); P = .007 |
| CV deathb | 3.81 (3.57-4.04) | 3.42 (3.25-3.58) | 3.55 (3.34-3.76) | –0.39 (–0.68 to –0.11); P = .007 | –0.26 (–0.57 to 0.05); P = .098 |
| Non-CV deathc | 4.67 (4.51-4.82) | 4.5 (4.38-4.62) | 4.51 (4.35-4.68) | –0.17 (–0.35 to 0.02); P = .085 | –0.15 (–0.37 to 0.07); P = .181 |
| Death due to HFd | 4.21 (3.99-4.42) | 3.89 (3.74-4.05) | 4.17 (3.98-4.35) | –0.31 (–0.57 to –0.05); P = .018P | –0.04 (–0.31 to –0.23); P = .775 |
| Death due to a cause other than HFe,5 | 4.21 (3.98-4.44) | 3.96 (3.8-4.11) | 3.72 (3.49-3.96) | –0.25 (–0.52 to 0.02); P = .068 | –0.48 (–0.81 to –0.16); P = .004 |
CV, cardiovascular; HF, heart failure; INR, international normalized ratio; LVEF, left ventricular ejection fraction; NT-ProBNP, N-terminal probrain natriuretic peptide.
Model adjusted for age, first admission for HF, branch block, Charlson comorbidity index, heart rate, urea, systolic blood pressure, hemoglobin, and NT–proBNP.
Model adjusted for age, first admission for HF, branch block, LVEF < 40%, Charlson comorbidity index, heart rate, urea, systolic blood pressure, hemoglobin, and NT-proBNP.
Model adjusted for age, LVEF < 40%, Charlson comorbidity index, heart rate, systolic blood pressure, and hemoglobin.
Of the 495 deaths recorded, 378 (76.4%) were attributable to cardiovascular causes. The crude rates for the specific causes of death by INR category are shown in Table 2.
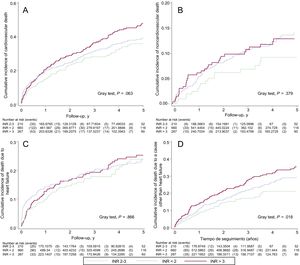
The cumulative risk of cardiovascular death was higher in patients with nontherapeutic INR values (Gray test, P = .063) and was particularly high in those with values above 3 (Figure 4). After the multivariate adjustment, however, although both subtherapeutic and supratherapeutic INR values were associated with a higher risk of cardiovascular death, the association was somewhat stronger for subtherapeutic values (differences in RMST at 5 years: RMSTINR<2 = –0.39 years (95%CI, –0.68 to –0.11); P = .007, and RMSTINR>3 = –0.26 years (95%CI, –0.57 to 0.05) years; P = .098) (Table 3).
The survival curves for the secondary endpoints are shown in Figure 5. The association between the risk of cardiovascular death and subtherapeutic INR values was stronger in the early follow-up period, as is also reflected in the time-dependent hazard ratios (Table 1 of the supplementary material). Compared with INR values in the therapeutic range, supratherapeutic values were associated with a sustained increased risk, but this did not reach statistical significance.
The crude rates for cause-specific mortality were low (Table 2 of the supplementary material). No significant differences were observed for death attributable to ischemic stroke, acute myocardial infarction, hemorrhagic stroke, or sudden death, although there were a higher number of deaths due to hemorrhagic stroke and sudden deaths in patients with INR values above 3. The cause of death was unknown in 84 patients (16%).
INR and Noncardiovascular DeathIn total, 117 deaths (23.6%) did not have a cardiovascular cause. The incidence of noncardiovascular death was higher in the subtherapeutic and supratherapeutic categories (Table 2), but the differences with patients in the therapeutic category were not significant (Gray test, P = .379) (Figure 4).
Because the assumption of proportional hazards was met for this criterion, the prognostic values of the different INR categories are presented as single hazard ratios in Table 4. The results confirm the lack of association between INR and the risk of noncardiovascular death identified in the RMST analysis (Table 3).
Risk Estimates for Different Analyses Meeting the Proportional Hazards Assumption for Subtherapeutic (INR < 2) and Supratherapeutic (INR > 3) Categories Compared With INR 2–3
| Criterion | Variable | sHR (95%CI) | P | Overall value, P | Harrell C statistic |
|---|---|---|---|---|---|
| Non-CV deatha | INR 2-3 | 1 | .362 | 0.666 | |
| INR < 2 | 1.48 (0.86-2.56) | .160 | |||
| INR > 3 | 1.44 (0.79-2.64) | .234 | |||
| Death due to causes other than HFb | INR 2-3 | 1 | .016 | 0.659 | |
| INR < 2 | 1.38 (0.96-1.98) | .084 | |||
| INR > 3 | 1.75 (1.19-2.59) | .005 |
95%CI, 95% confidence interval; CV, cardiovascular; HF, heart failure; INR, international normalized ratio; LVEF, left ventricular ejection fraction; sHR, subhazard ratio.
The number of deaths due to bleeding was very low (Table 2 of the supplementary material) and no differences were observed in cumulative risk between the 3 INR categories (Gray test, P = .949).
INR and Death Due to Heart FailureA total of 230 deaths were attributed to heart failure (46.5%). Although the crude incidence rate was higher in patients with nontherapeutic INR values (Table 2), no significant differences were observed for cumulative risk between the 3 categories (Gray test, P = .866) (Figure 4).
The category associated with the highest risk of mortality due to heart failure was the subtherapeutic category, which was mostly attributable to early events. In the multivariate RMST analysis, only patients with subtherapeutic INR values had an increased risk of death due to heart failure: RMSTINR<2 = –0.31 years (95%CI, –0.57 to –0.05) (P = .018) (Table 3 and Figure 5). The time-dependent hazard ratios (Table 1 of the supplementary material) showed a pronounced and significant increase in risk in the subtherapeutic category, but this decreased rapidly and lost its significance after the third month of follow-up.
INR and Death Due to a Cause Other Than Heart FailureThere were marked differences between the 3 INR groups in terms of the cumulative incidence of death due to causes other than heart failure, with the highest rate observed in the supratherapeutic category (Gray test, P = .018) (Figure 4).
In the multivariate analysis (Table 3), patients with supratherapeutic INR values had a considerably increased risk of death due to a cause other than heart failure compared with patients with values in the therapeutic range: RMSTINR>3 = –0.48 (95%CI, –0.81 to –0.16) (P = .004) (Figure 5). The assumption of proportional hazards was also met for this criterion and the prognostic values of the different INR categories are thus shown as a single hazard ratio in Table 4, supporting the results of the RMST analysis.
DISCUSSIONThe main finding in this study is that an INR value outside (above or below) the optimal therapeutic range in patients with acute heart failure and NVAF on VKA therapy on admission to hospital is independently associated with an increased risk of mortality during long-term follow-up.
Heart failure and atrial fibrillation often coexist as they share risk factors and pathophysiologic mechanisms.15 The presence of heart failure significantly increases the risk of thromboembolic events in patients with atrial fibrillation and is itself an indication for long-term oral anticoagulant therapy.3 It is not easy, however, to manage oral anticoagulant therapy in patients with heart failure and atrial fibrillation.7,16 Several factors limit effective control, such as polypharmacy, frequent rehospitalization, renal or hepatic dysfunction, and hemostatic alterations linked to heart failure7–9,17 and in particular acute heart failure.7
In the present series, over 80% of patients had an altered INR value on hospital admission. Possible explanations include factors that may have a negative effect on VKA metabolism, such as hepatic ischemia and/or tissue hypoxia due to reduced hepatic flow in patients with congestion.18 INR values in patients with heart failure have also been correlated with degree of systemic inflammation8 and hemodilution associated with systemic venous congestion.19 The data from our study partly support the above hypotheses. In our series, altered INR values were significantly associated with elevated levels of certain biomarkers, including carbohydrate antigen 125 and N-terminal probrain natriuretic peptide. We found no association, however, between altered INR values and liver function marker levels.
The pathophysiologic mechanisms underlying the association between INR on hospital admission and risk of mortality are largely unknown. It is noteworthy, however, that the factors mentioned above as potential causes of altered INR values are all negative pathophysiologic and prognostic factors in heart failure. Nevertheless, the persistence of the association between altered INR and mortality after adjustment for confounders in the multivariate analysis, together with the strength of this association in the early phases of follow-up, suggest a possible causative link. In a recent study of 294 patients with acute heart failure who were not on anticoagulant therapy, Okada et al.9 observed that altered INR values on admission were independently associated with lower survival at 1-year of follow-up. As in our study, these values were also associated with a worse neurohormonal activation profile. It should be noted, however, that the patients in our series were all on VKA therapy, and as such, INR was not only a biomarker, but also a modifiable treatment goal within each patient's anticoagulant management program.
Our exploratory analysis did not identify any clear associations between INR values and specific causes of death within the different INR categories, although it did reveal some interesting associations. Patients in the subtherapeutic category (INR < 2), for example, had a greater risk of dying of heart failure. Clinical deterioration and advanced disease in heart failure patients have been linked to increased drug interactions and decreased treatment adherence. Both of these situations have been associated with poor prognosis in heart failure20 and could partly explain our findings. Patients in the supratherapeutic category (INR > 3) had a higher risk of dying of a cause other than heart failure. Although the reasons for this association are speculative, INR values above 3 were more common in patients with preserved left ventricular ejection fraction. As is known, heart failure with preserved ejection fraction is a multifactorial syndrome characterized by complex interactions between cardiovascular disorders and different comorbidities,21 and patients are more likely to develop other diseases and die of causes other than heart failure.22 At the same time, valvulopathy was more common in the supratherapeutic category, which could also partly explain the higher mortality in this subgroup.
A recent study showed that a patient-specific tailored oral anticoagulant therapy intervention significantly improved control in heart failure patients.23 It is not known, however, what effect good control of oral anticoagulant therapy has on mortality risk in this setting.
One option that does not require INR monitoring is to use direct anticoagulants. Landmark clinical trials comparing direct anticoagulants and VKAs in patients with NVAF have included over 25 000 patients with heart failure, 13 251 of whom were treated with direct anticoagulants.5 In the subgroup of heart failure patients, direct oral anticoagulants reduced the incidence of stroke or systemic embolism and major and intracranial bleeding, but had a neutral effect on all-cause and cardiovascular mortality.4 Detection of altered INR on hospital admission could help identify a subgroup of patients in whom direct anticoagulants could be particularly beneficial compared with VKAs. More evidence, however, is needed as different criteria have been used to define heart failure and none of the trials have studied patients with acute decompensated heart failure.
INR did not meet the assumption of proportional hazards for its association with mortality risk. Analysis of RMST differences has been recommended as a suitable alternative for evaluating differences in survival attributable to a study variable over a given period, and its use is increasing.12,13 The findings of our study show that, following multivariate adjustment for confounders, subtherapeutic and supratherapeutic INR values were respectively associated with a 6-month and 5-month reduction in mean 5-year survival compared with values in the therapeutic range. Risk attributable to INR on admission, however, was greater in the early follow-up period, particularly for patients with a value of less than 2. The association between supratherapeutic values and mortality risk, by contrast, remained more constant during long-term follow-up. More studies are needed to explain the pathophysiologic mechanisms behind these findings and to understand their implications for treatment.
LimitationsThis study has several limitations. First, as an observational, single-center study, its results may have been influenced by several biases and aspects inherent to daily practice. Second, we were missing data on time in therapeutic range on admission and on subsequent INR levels, meaning that we have no information on level of anticoagulant therapy control beyond the acute episode. Third, assignment of specific causes of death in observational studies is complicated and has evident limitations.11 In this regard, the low incidence of deaths attributable to certain cardiovascular events, together with the presence of deaths due to unknown causes, makes it difficult to draw any firm conclusions about specific causes of death attributable to INR. Finally, INR was not measured at the same time as other variables (eg, echocardiographic variables).
CONCLUSIONSINR values below or above optimal therapeutic values in patients with heart failure and NVAF on hospital admission for acute decompensated heart failure are independently associated with an increased risk of mortality during long-term follow-up.
CONFLICTS OF INTERESTNone declared.
- –
The prognostic value of INR values in patients on VKA therapy who experience acute decompensated heart failure is unknown.
- –
Overall, 81% of patients with heart failure and NVAF on VKA therapy had an INR value outside the therapeutic range during acute decompensated heart failure.
- –
Subtherapeutic and supratherapeutic INR values are independently associated with an increased risk of mortality during follow-up.