Drug-eluting stents are useful for preventing restenosis, but the patho-physiological processes involved in the proliferative response after implantation are still not known in detail. The aim of this study is to compare the coronary vascular histomorphometry after implanting drug-eluting stents and bare metal stents in a swine model.
MethodsSixty stents were randomly implanted in 20 Large White female pigs with a ratio of baremetal/drug-eluting stents of 1:2. After 28 days, euthanasia and histomorphometry were performed. We defined the vessel injury score in accordance to whether the internal elastic lamina was intact or ruptured.
ResultsThere were no differences between drug-eluting stents and bare metal stents in the intact internal elastic lamina group regarding neointimal area or % restenosis (1.3 [1.1-2.2]) vs 2.0 [1.3-2.5] mm2; P=.6; and 14.0 [12.1-20.8] vs 22.2 [14.1-23.3] %; P=.5). We assessed statistically significant differences for the ruptured internal elastic lamina group, (neointimal area 1.2 [0.8-2.0] vs 2.9 [2.3-3.7] mm2; P=.001 and % restenosis 16.63 [11.2-23.5] vs 30.4 [26.4-45.7] %; P=.001).
ConclusionsIn our swine model, we did not find any differences between proliferative response of drug-eluting stents and bare metal stents when the internal elastic lamina is intact; differences are only found when vascular injury is deeper.
Keywords
Healthy coronary arteries in a swine model is currently considered the standard for evaluating different platforms, polymers, and drugs for stents, given the anatomical and physiological similarity with the human model, and because its biological processes develop rapidly.This similarity also extends to the myocardial tissue repair system,1, 2, 3 and the proliferative response or restenosis following coronary stent implantation.3, 4, 5
This proliferative response is the main determining factor for long-term stent outcomes, and has been considerably reduced with the advent of drug-eluting stents (DES). Despite the encouraging results from clinical trials,6, 7, 8, 9, 10 due to their mechanism of action DES have lately been reported to provoke high rates of delayed thrombosis secondary to problems such as withdrawal from antiplatelet therapy,11 delayed malapposition,12 lack of endothelisation13 or chronic inflammation.14, 15 This is why pre-marketing experimental models examining the endothelial reaction and intimal reaction to new devices have become more important.
In this respect, preclinical assessment must include a histomorphometric study of the stents 28 days after implantation. Factors that may modify the biological response to different intravascular devices in this experimental model must actually be taken into account before transferring the experimental study conclusions to human beings.
Although the relationship between vessel injury and restenosis has been described,16 preclinical studies do not always provide this data given that is difficult to acquire such information.
The aim of the study was to examine the differences between conventional and DES in the swine model, and associate them with the degree of vessel injury induced during implantation.
METHODS SampleWe selected 20 two-month-old, Large White, female pigs, weighing 25-30kg. We handled them in accordance with current European Union legislation on animal testing, following the guidelines established in the Spanish RD 223/98 of 14 March on protecting animals used for testing and other scientific purposes.
We implanted several stents in the proximal segments of the three coronary arteries, obtaining a sample size of 60 treated segments.
Furthermore, we extracted 22 non-treated arterial segments to use them as a control group for the histomorphometric variables of the healthy arteries.
Procedure Drug Treatment and MonitoringThe day before the procedure, the animals were isolated and oral antiplatelet medication was initiated with 100mg of acetylsalicylic acid and a 300mg loading dose of clopidogrel. Maintenance doses of 100mg and 75mg, respectively, were then administered every 24h throughout the follow-up time, as well as 20mg/kg of ciprofloxacin as antibiotic prophylaxis.
On the day of the procedure, the animals were administered 0.35mg/kg of midazolam, 5mg/kg of ketamine and 0.02mg/kg of intramuscular atropine. We checked that the sedation level was adequate and anaesthetic propofol was administered at a dose of 2mg/kg-4mg/kg by slow intravenous infusion. We inserted an endotracheal tube and connected the animals to volume-controlled mechanical ventilation, maintaining anaesthesia with isoflurane at 2%.
We monitored the animals throughout the procedure by means of pulse oximetry, electrocardiogram and invasive arterial pressure measurement. Intraoperative medication was 5000 IU of sodium heparin, 2.5mg/kg of intramuscular cefquinome as a prophylactic antibiotic therapy, and 0.01mg/kg of intramuscular buprenorphine as an analgesic measure.
ImplantationAfter we had surgically accessed the right common carotid artery, we passed a 6F introducer sheath into it and performed selective catheterisation of the coronary ostia with a modified Amplatz guidewire catheter, designed especially for this study (Iberhospitex, S.A.), which allows the same catheter to be passed through both ostia. The guidewire was passed towards the distal bed and the stent was implanted in line with the previously designed randomised assignment table.
All of the stents were the same diameter and length (3.5 mm × 18 mm). We applied enough pressure to obtain a balloon/artery diameter ratio of between 1.2:1 and 1.3:1 (between 12 and 16 atmospheres. The nominal pressure for the device was 10 atmospheres). We implanted 60 stents between two groups:
– CS GROUP: 20 arterial segments with conventional stents.
– DES GROUP: 40 arterial segments with paclitaxel-covered stents.
Once the 28-day follow-up period was complete, we performed euthanasia administering T61® intravenous agent (0.3ml/kg; Intervet, Spain). Prior to euthanasia we sedated the animals with midazolam (0.35mg/kg) and ketamine (5mg/kg) both administered via intramuscular injection. We then supplied a dose of sodium heparin (10 000 IU) to make it easier to clean the preparations.
Phosphate-buffered saline was perfused into the coronary arteries in situ, at an approximate pressure of 100-110mmHg. Following this, we dissected the coronary arteries from the epicardial surface, respecting margins 5mm distal and proximal to the stent. Then, we then fixed them in 10% buffered formalin over 24-48h at room temperature.
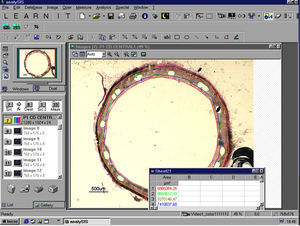
Histomorphometric AnalysisTo perform the histological study, the sections were embedded in resin, and following the microtome cross-section, they were stained using the Masson's trichrome and Verhoeff-Van-Gieson's techniques for elastic fibres. We then conducted the histomorphometric analysis at a constant magnification of ×15, using a trinocular stereo-microscope (Olympus SZ61) with an integrated digital camera connected to the computer system. We used the Image-Pro Plus 4.0 image analysis programme (Media Cybernetics S.A.) to obtain the images (Figure 1).
Figure 1. Example of histomorphometric analysis, computerised area quantification. Screenshot of the Image-Pro Plus 4.0 (Media Cybernetics, S.A.), while analysing one of the implanted stent samples.
We obtained the following direct or primary measurements:
– EEL: area within the external elastic lamina.
– IEL: area delimited by the internal elastic lamina.
– LA: area occupied by the arterial lumen area.
We used these area data to calculate four new indirect or secondary measurements:
– TMA: tunica media area (EEL-IEL).
– NA: neointimal area (IEL-LA).
– Percentage (%) of restenosis per area: [1- (LA / IEL)] × 100 (percentage of neointima that occupies the IEL area.
– NA/EEL index (ratio between the NA and the EEL area).
We also calculated the degree of vessel injury or Injury Score (IS) of each stent strut for each section, only considering whether the IEL was ruptured or not. We used modified Schwartz et al.16 and Gunn et al.17 injury scores, defining IS-1 as intact internal elastic limiting lamina, and IS-2 for its rupture.
All of the histomorphometric assessment was performed by a qualified anatomical pathologist who was unaware of which stent had been implanted in each of the sections.
Statistical AnalysisThe univariate descriptive statistics were resolved with central tendency and dispersion measures when the data were quantitative, and with frequencies/percentages when they were qualitative.
The normality of variables was calculated using the Kolmogorov-Smirnov test.
We used parametric tests and non-parametric tests, where necessary, for the bivariate statistical analysis. We used the chi-square test to compare the qualitative variables in the parametric tests, and the ANOVA test to compare quantitative variables with the a posteriori Tukey test to perform multiple comparisons. Homogeneity of the variances was calculated using the Barlett's test. We considered differences to be statistically significant for all of the analyses when P<.05.
Multivariate AnalysisIn order to add greater consistency to the data, the correlation and significance of the various histomorphometric variables and their relation to the degree of vessel injury were checked against a linear regression model.
We used the multivariate adaptive regression splines (MARS) to examine the influence that discreet variables had on the degree of restenosis and NA size.
We used SPSS version 15.0 (http://www.spss.com/es/) for Windows and Minitab version 15.0 (http://www.minitab.com/es-ES/default.aspx).
RESULTS ProcedureThe primary success rate for the procedure was 100%. Two animals died (6 stent-treated segments) a few hours after the procedure due to arrhythmia. The remaining 18 animals completed the 4-week follow-up period.
Furthermore, we removed 10 arterial segments from the analysis: 7 due to over-stretch injury causing the tunica media (3 cases) and adventitia layers (4 cases) to rupture, with an extensive granulomatous reaction. Another 3 segments were damaged during the resin casting process.
Therefore, the final analysis was conducted on 18 animals (44 treated arterial segments), plus 22 untreated arterial segments (healthy controls).
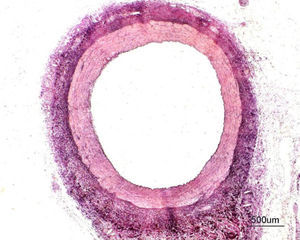
HistomorphometryControl group: We extracted 22 artery segments proximal to the implant areas for the control group (Figure 2). Given that no stents were present, the IEL and LA variables were the same. Table 1 summarises the characteristics observed.
Figure 2. Histological cross-section of a healthy swine coronary artery (proximal segment). Haematoxylin and eosin and Van-Gieson staining (×10); three arterial layers can be seen. The internal and external elastic limiting laminae can also be observed (in black).
Table 1. Histomorphometric Characteristics of a Healthy Swine Coronary Artery (n=22).
| Variables | Median [interquartile range] |
| EEL (mm2) | 3.64 [2.94-4.45] |
| IEL (mm2) | 2.44 [1.83-2.68] |
| TMA (mm2) | 1.36 [1.00-1.76] |
| LA (mm2) | 2.44 [1.83-2.68] |
EEL, external elastic lamina; IEL, internal elastic lamina; LA, lumen area; TMA, tunica media area.
Study group: We observed that the vessel size of the treated segments increased significantly in comparison to the control segments. There were no significant differences in the degree of vessel injury among the treated artery types (Table 2). The IEL was intact (IS-1) for 55% of cases, and ruptured (IS-2) for 45%.
Table 2. Contingency Table of the Vessel Injury Depending on the Treated Artery.
| Circumflex artery | Right coronary artery | Anterior descending artery | Total | |
| IS-1 | 6 | 12 | 6 | 24 |
| IS-2 | 8 | 4 | 8 | 20 |
| Total | 14 | 16 | 14 | 44 |
IS: Injury Score.
χ2=4.243; P=.12.
When we compared CS with DES, regardless of the degree of vessel injury, the paclitaxel-eluting stents had a significantly smaller NA, less restenosis, and a larger LA that the bare stents (NA 1.26 [0.99-2.09] vs 2.54 [2.26-3.14] mm2; P<.001. Restenosis 15.40 [12.13-21.44] vs 29.00 [23.54-38.66] %; P<.001; LA 7.84 [6.87-8.24] vs 5.96 [5.14-6.94]; P=.006).
When we analysed the degree of vessel injury (Figure 3) the segments with the deepest vessel injury (IS-2) had a smaller vessel lumen and greater percentage of restenosis than the IS-1 group (Table 3). Considering the type of stent implanted, we did not find any significant differences with regards the neointimal proliferation parameters for the IS-1 group, but we did observe statistically significant differences for the IS-2 group (Table 4).
Figure 3. Histology. Cross-sections with haematoxylin and eosin and Van-Gieson staining (×100) on the apposition area of one of the struts to identify the internal elastic lamina. Left: type 1 vessel injury (intact internal elastic lamina). Right: type 2 vessel injury (ruptured internal elastic lamina). EEL, external elastic lamina; IEL, internal elastic lamina.
Table 3. Histomorphometric Characteristics of the Treated Segments Depending on Vessel Injury.
| IS-1 (n=24) | IS-2 (n=20) | P | |
| EEL(mm2) | 11.04 [10.24-12.27] | 10.99 [9.48-12.13] | .005 |
| IEL (mm2) | 9.40 [8.69-10.35] | 8.57 [7.54-9.36] | .010 |
| TMA (mm2) | 1.81 [1.47-2.07] | 1.61 [1.43-1.70] | .044 |
| NA (mm2) | 1.37 [1.13-2.32] | 2.30 [1.24-3.06] | .062 |
| LA (mm2) | 8.03 [7.29-8.36] | 6.03 [4.98-7.18] | .001 |
| IP (atmospheres) | 14.00 [12.25-15.75] | 14.00 [13.00-16.00] | .461 |
| NA/EEL | 0.13 [0.10-0.19] | 0.22 [0.14-0.31] | .003 |
| Restenosis (%) | 15.27 [12.09-22.34] | 26.38 [17.33-35.17] | .003 |
EEL, external elastic lamina; IEL, internal elastic lamina; IP, implant pressure; IS, injury score; LA, lumen area; NA, neointimal area; NA/EEL, ratio between neointimal area and external elastic area; TMA, tunica media area.
The variables’ results are expressed as median [interquartile range].
Table 4. Histomorphometric Characteristics of the Treated Segments Depending on Vessel Injury and Type of Stent Implanted.
| IS-1 | P | IS-2 | P | |||
| DES (n=20) | CS (n=4) | DES (n=9) | CS (n=11) | |||
| NA (mm2) | 1.32 [1.10-2.15] | 2.02 [1.26-2.49] | .60 | 1.22 [0.80-1.95] | 2.90 [2.27-3.66] | .001 |
| LA (mm2) | 7.91 [7.29-8.35] | 8.21 [6.51-8.60] | .99 | 7.26 [5.17-8.15] | 5.40 [4.93-6.48] | .054 |
| Restenosis (%) | 13.96 [12.09-20.80] | 22.23 [14.07-23.27] | .45 | 16.63 [11.16-23.47] | 30.44 [26.38-45.74] | .001 |
CS, conventional stent; DES, drug-eluting stent; IS, Injury Score; LA, lumen area; NA, neointimal area.
The variables’ results are expressed as median [interquartile range].
We used a linear regression model for the restenosis variable, calculated according to IEL, EEL, TMA, NA, LA, NA/EEL and the inflation pressure (IP). Once we had eliminated the variables with the highest P quotients, we obtained an optimum model including the variables TMA, LA and NA/EEL with an R2 of 98.9% (which is the same as the model performed with seven variables).
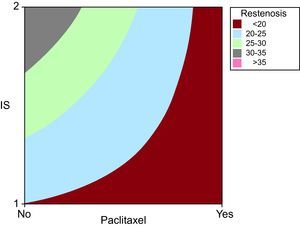
We analysed how paclitaxel influenced restenosis, observing that when it was present, the degree of restenosis independently reduced. However, its effect was more apparent when the degree of vessel injury was greater (Figure 4).
Figure 4. Contour graph created using the Minitab 15.0 statistical software with the variables Injury Score and paclitaxel compared against the restenosis variable. It shows the proportional relationship between vessel injury influence during implantation and the presence or absence of drugs in the stent on the degree of neointimal proliferative response. We can see that the higher restenosis values belong to the drug-free stents and IS-2 group. Note that although restenosis can have any value within the range for drug-free stents, drug-eluting stents only have the lowest restenosis values. IS, Injury Score.
We also performed a MARS model for “restenosis” in relation to EEL, IEL, LA, TAM, NA, NA/EEL, IP, stent type, IS and treated artery type. This model can process both continuous and discreet variables correctly. After ruling out variables, the MARS only found the variables NA/EEL, IEL, NA TAM and IS as significant.
DISCUSSION ModelThere is currently no ideal animal model for human cardiovascular disease, although the vessel injury and neointimal proliferative response in the swine model of coronary artery lesions and in the rabbit model of iliac artery lesions are similar to human restenosis processes.16, 18 Experimental studies suggest important similarities between inflammation, vessel injury and neointimal growth in these models and in human coronary arteries, even though the vessel injury caused to these animal models is different from that caused in human atherosclerotic arteries.19, 20 The histological results from this study confirm that the proliferative response observed is proportional to the vessel injury produced by stent over-stretching.
Vessel InjuryThe most important finding from this study is the relationship between the depth of the vessel injury during stent implantation and the proliferative reaction of the intima, or between the IS and restenosis, which in essence is the same.
Our sample's vessel injury is graded IS-1 for 55% and IS-2 for 45%. The data that we have found are in accordance with the data published by Gunn et al.17 which showed that complete compression of the media layer with the IEL being intact was even more common than the deeper vessel injuries.
According to this theory, compression of the tunica media must be considered as the most important “overall stimulus” following moderate degree of vessel injury. Furthermore, the most significant point that we found was that it had a positive correlation with the intensity of neointima formation response. Our results therefore confirm that the variability of the proliferative response is proportional to the degree of vessel injury, even though this response is reduced for the DES group.
It is thought that the possible mechanisms why the tunica media layer compression induces neointimal proliferation is because stent impact compresses the smooth cellular muscles around each strut, leading to atrophy of the media and the dead cells, due to necrosis or apoptosis. They could then trigger the biological wound healing processes which lead to neointima formation. Another possibility is that the compression is transferred cell to cell by adhesion molecules or intracytoplasmic integrins that may activate kinases or metalloproteases, leading to neointimal proliferation and/or connective tissue formation.21, 22, 23
Efficacy of Drug-Eluting StentsThe DES group's response was significantly less proliferative than the CS group, meaning that the vessel lumen increased, and the NA and restenosis decreased. Furthermore, when we compared the stents with and without paclitaxel, in accordance with the degree of vessel injury, there were only significant differences in the proliferation variables for the subgroup with more vessel injury. As such, when we compared each group separately for vessel injury, the CS group showed significant differences for the proliferation variables but the DES group did not.
Eshtehardi et al.24 recently published a study on humans following a DES implant. It showed that there was no relationship between the degrees of vessel injury analysed using intravascular ultrasound and the proliferative response for this type of stent. This supports the conclusion that paclitaxel efficiently inhibits the vascular aggression-triggered proliferative response, in such a way that no restenosis differences between groups with or without IEL rupture are found, given that it is extremely weakened.
We can confirm that the paclitaxel-covered stent is significantly better than the bare stent regarding antiproliferative efficacy, having 55% neointimal hyperplasia reduction and 52% restenosis reduction. However, when the IEL remains intact, DES does not seem to be any better than CS.
When we applied the multivariate analysis models to the data obtained in this model, we found that the DES proliferative response to vessel injury does not have the same degree of proportionality as the conventional stents. Therefore, the proliferative response (defined by the IEL rupture) is marked from a cross-section point, not by the degree of vessel injury, but by the drug's antiproliferative efficacy. This observation appears to not have included clinical and considerable efficiency implications. Therefore, although it is not a common situation in clinical practice, when the IEL is intact there would not be any significant additional benefit with DES use.
Taking these data into account when designing preclinical experiments, the degree of vessel injury during implantation must be examined thoroughly to be able to consider that the proliferative reaction of the intima may have been modified.
Some authors suggest that the intact IEL acts as a barrier and stops smooth muscle cells or progenitor cells from migrating, and that if this barrier is broken, the function is lost.25, 26 Until recently, it was accepted that restenosis conditioned a phenotypic change in smooth muscle cells, from contractile cells to cells with synthesis capacity. However, there is increasingly more evidence that the cellular components of the neointima originate from the adventitial microfibroblasts26, 27, 28, 29 or from bone marrow stem cells which migrate through the adventitia to the media and intima layers.30, 31 It therefore seems logical to imagine that the closer this area is to the vascular aggression, the greater the proliferative stimulus.
It is possible that these observations may lead future DES research towards searching for different drug-eluting mechanisms which make it more effective.
Study LimitationsThis study presents limitations owing to restenosis experimental models for large mammals, i.e. using a small number of animals, only using female animals to avoid arrhythmia-associated mortality and the fact that a lesion had to be induced on a healthy artery. Although the proliferative response obtained allowed us to examine the drug's activity, we do not know if this effect would be the same for coronary arteries with a high content of atherosclerotic material, where IEL fragmentation areas appear spontaneously as part of the overall plaque inflammation process, especially for vulnerable plaque,32 and other biochemical mediators participate in the vessel repair process. Furthermore, patients with atherosclerosis present with other molecular and genetic factors, such as a greater presence of oxidised low-density lipoproteins, hyperglycaemia or arterial hypertension, which could interfere directly with the process. Despite this, the coronary swine model continues to be recommended by consensus for evaluating this type of device.
CONCLUSIONSPaclitaxel-covered coronary stents significantly reduce restenosis compared to bare stents in a swine model. This difference in proliferative response intensity is established in cases of deep vessel injury with rupture of the IEL during implantation. However, this benefit can not be extended to cases where the IEL remains intact.
These data should be taken into account when designing and analysing future experimental studies aimed at evaluating the antiproliferative power of the different cytostatic drugs used in preventing intracoronary stent restenosis.
CONFLICT OF INTERESTNone declared.
Received 5 November 2010
Accepted 17 March 2011
Corresponding author: Sección de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Hospital de León, Altos de Nava s/n, 24071 León, España. alejandrodiego@secardiologia.es