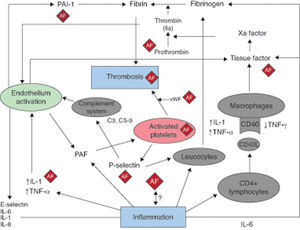
Inflammation is known to play an important pathogenic role in several cardiovascular diseases. Evidence accumulated over the past two decades supports a key role for inflammation in all phases of the atherosclerotic process, from endothelial dysfunction and plaque formation through to its progression and, ultimately, the thrombotic complications that lead to the acute coronary syndrome.1 Activated inflammatory cells (such as neutrophils, lymphocytes, monocytes, and resident macrophages), pro-inflammatory cytokines, and activated platelets are important players in this scenario. More recently, findings in clinical and experimental studies have suggested a possible role of inflammation in the pathogenesis of atrial fibrillation (AF) and –in particular- its thrombotic complications (Figure 1).
Figure 1. The relationship between atrial fibrillation, inflammation, and thrombosis. The AF boxes indicate areas where atrial fibrillation can affect or is affected by the mechanisms linking inflammation and thrombosis, as described in the text. AF, atrial fibrillation; IFN, interferon; IL, interleukin; PAI-1, plasminogen activator inhibitor-1; TNF, tumor necrosis factor; vWF, von Willebrand factor.
Atrial fibrillation and thrombosisIt is established that AF is associated with atrial thrombus formation and peripheral embolization, which often leads to the occurrence of stroke or other major thromboembolic complications. The latter represents a major clinical problem as, on average, the risk of thromboembolism-related stroke risk is over 2% per year in AF patients. During episodes of fast AF, blood flow is turbulent and much slower in the atria, particularly in the left atrial appendage, which is a major site of thrombus formation in AF patients. In relation to thrombus formation in this scenario, the question has emerged as to whether turbulence and relative flow stasis in the atria can explain the whole process. Current evidence, however, suggests that formation of thrombi in AF is the result of several variables. It is thought that the components of the “Virchow's triad” (ie, blood coagulation status, vessel wall-related factors, and a reduced blood flow) are principally responsible. Blood stasis, a hypercoagulable state, and endothelial dysfunction are all present in patients with AF and, indeed, most of the current strategies for prevention of stroke in AF patients are based on tackling both the problem of flow “turbulence” and the procoagulant state present in AF. To avoid atrial blood stasis, attempts are made to revert AF to sinus rhythm with the use of electrical or pharmacological cardioversion and, more recently, pulmonary vein isolation. Interestingly, no significant prognostic improvement has been achieved with this approach compared to rate control. Regarding the hypercoagulable state, anticoagulant therapy has been shown to be more effective than placebo or antiplatelet agents to prevent thromboembolic complications in AF. However, the third factor in Virchow's triad, ie, problems with the biology of the vessel/vascular tissue, typically endothelial dysfunction, has not yet been addressed fully as a potential therapeutic target. Could this be at least one of the reasons why some patients (1.1% to 1.7% per year) develop some form of systemic embolism or suffer a stroke despite the use of anticoagulant therapy?
Inflammation and thrombosisSeveral studies have suggested a link between thrombogenesis and inflammation. A relationship between these two entities has been identified in different clinical scenarios where the inflammatory process and coagulation abnormalities are clearly interlinked, for example in patients with septic shock and disseminated intravascular coagulation, and also in the context of deep venous thrombosis. In the acute coronary syndrome, inflammation also seems to regulate the thrombogenicity of the atheromatous plaques through the release of tissue factor and other molecules by plaque macrophages.2 Platelet aggregation and an impaired response of the natural anticoagulant systems also favors thrombus formation when the fibrous cap of the “culprit” plaque undergoes fissuring.3 Molecular mechanisms that could explain a link between inflammation and thrombosis are becoming apparent.2 C-reactive protein (CRP), an acute-phase reactant and a biomarker for cardiovascular risk, has been suggested to play a pathogenic role in several cardiac conditions.2 Inflammation is known to upregulate CRP expression and to modulate platelet function. Very importantly, it also affects the extrinsic blood coagulation cascade and the fibrinolytic system, enhancing the thrombotic response to vascular injury in vivo.2
Several studies suggest that the complement system (CS) may serve as a link between inflammation and thrombosis and reveal specific interactions between complement proteins and platelets. The CS was shown to induce platelet activation and aggregation as well as potentiate thrombin-induced platelet secretion and aggregation. At the same time, platelets can activate the CS via P-selectin.4
Proinflammatory cytokines have been related to a prothrombotic state in different clinical settings. For example, interleukin (IL)-6 induces the expression of tissue factor, fibrinogen, factor VIII, and von Willebrand factor.5 These have been linked to increased platelet aggregation, sensitivity to thrombin, and both endothelial activation and endothelial cell damage. Moreover, increased IL-6 levels lowers the concentration of the natural inhibitors of hemostasis such as antithrombin and protein S.5 From a clinical perspective, it is relevant that both tissue factor and stroke risk are independently associated with IL-6 levels.6 Other procoagulant molecules, such as fibrinogen, and also plasma viscosity were shown to be independently associated with inflammation.7 CD40 ligand (L), a member of the tumor necrosis factor (TNF) superfamily, is primarily expressed on activated CD4+ T lymphocytes but is also found in a soluble form. CD40L was originally described on T cells, but its expression has since been found on a wide variety of cells, including platelets, mast cells, macrophages, NK cells, B lymphocytes, vascular smooth muscle cells, and endothelial cells. Soluble CD40L binding to its receptor CD40 on the leukocyte can also induce tissue factor expression.8 Vascular smooth muscle cells represent a link between inflammation and thrombosis, as the local thrombotic stimulation of smooth muscle cells in the arterial wall can amplify the inflammatory response and increase fibrinogen and plasminogen activator inhibitor-1 levels in the circulation.2 Of importance, AF represents a condition where many, if not all, of these mechanisms linking inflammation and thrombosis can be operative (Figure 1).
Inflammation, thrombosis and atrial fibrillationInflammation has been suggested to be a pathogenic factor in AF, as studies have shown a correlation between inflammatory markers and incidence of AF. In 190 patients with nonrheumatic AF undergoing transesophageal echocardiography, Maehama et al.9 observed that raised circulating CRP levels correlated with the presence of left atrial thrombi.9 Clinical outcomes, ie, ischemic stroke and mortality, appear also to be influenced by inflammation (increased high-sensitivity CRP concentrations) according to findings in a large prospective study involving 7901 patients.10 Concordantly, a study involving 880 patients with AF who received treatment with aspirin (325mg/d, alone or combined with warfarin), showed that CRP levels increased in a positive fashion with increasing stroke risk as measured by CHADS2 and NICE (National Institute for Health and Clinical Excellence) risk criteria, as well as with prognosis (mortality and vascular events, but not stroke), suggesting a positive correlation between inflammation and other established stroke risk factors.11 Surprisingly, in the study, soluble CD40L levels were negatively associated with stroke risk and were not related to stroke events, vascular events, or all-cause mortality during a mean follow-up of 453 days.11 The authors argue that these controversial findings may be due to the fact that high levels of sCD40L are indicative of the presence of “symptomatic” atherosclerotic plaques, whilst CRP is a marker of “pan-vascular” inflammation. Moreover, given that thrombogenesis in AF may be more coagulation factor-related, rather than platelet-related, the more limited relationship of sCD40L to stroke risk in AF seems plausible. Another study12 reported in 150 subjects with AF unrelated to valve heart disease that CRP concentration was significantly associated with the occurrence of thrombosis but not with left atrial appendage flow velocity, as assessed by echocardiography. This finding suggests that inflammation can constitute an independent pathogenic factor for thrombogenesis in this setting. Supporting this notion is the fact that tissue factor was found to be overexpressed in the endothelium of vessels obtained from tissue containing inflammatory cells and in the denuded matrix of the endocardium in patients with nonvalve heart disease AF and thromboembolism, compared with control subjects.13 However, despite the evidence suggesting a link between inflammation and thrombogenesis in AF, results have been reported which failed to show a correlation between a procoagulant status and inflammation in AF.14, 15
Taken together, the results of the studies mentioned above appear to suggest that systemic inflammation may contribute to thrombus formation and possibly to the development of stroke and other forms of thromboembolism in AF, but there are controversial results at present. The link between inflammation and thrombosis could provide an explanation to the clinical finding that some patients suffer thrombotic events even when receiving appropriate anticoagulant therapy, as in such cases CRP levels were found to be significantly higher, compared to those in patients without thrombosis.9 However, much of the information available at present is based on relatively small cohorts and there is a lack of mechanistic studies. Whether high CRP levels are a useful marker to identify AF patients who are at a higher risk of thrombosis warrants further investigation.
The link between inflammation and thrombogenesis is currently beyond dispute; however, the issue that still needs to be resolved is whether this link is of clinical relevance in AF. Addressing the question as to whether inflammation represents a true pathogenic factor in this setting and whether biomarkers of inflammation could be used to detect patients at a higher risk is of utmost importance, and further research in this field should therefore be strongly encouraged.
Conflicts of interestNone declared.
.
Corresponding author: Cardiovascular Sciences Research Centre, St. George's University of London, Cranmer Terrace, London SW17 0RE, United Kingdom. jkaski@sgul.ac.uk