Hypertrophic cardiomyopathy is a frequent cause of sudden death. Clinical practice guidelines indicate defibrillator implantation for primary prevention in patients with 1 or more risk factors and for secondary prevention in patients with a history of aborted sudden death or sustained ventricular arrhythmias. The aim of the present study was to analyze the follow-up of patients who received an implantable defibrillator following the current guidelines in nonreferral centers for this disease.
MethodsThis retrospective observational study included all patients who underwent defibrillator implantation between January 1996 and December 2012 in 3 centers in the province of Barcelona.
ResultsThe study included 69 patients (mean age [standard deviation], 44.8 [17] years; 79.3% men), 48 in primary prevention and 21 in secondary prevention. The mean number of risk factors per patient was 1.8 in the primary prevention group and 0.5 in the secondary prevention group (P=.029). The median follow-up duration was 40.5 months. The appropriate therapy rate was 32.7/100 patient-years in secondary prevention and 1.7/100 patient-years in primary prevention (P<.001). Overall mortality was 10.1%. Implant-related complications were experienced by 8.7% of patients, and 13% had inappropriate defibrillator discharges.
ConclusionsIn patients with a defibrillator for primary prevention, the appropriate therapy rate is extremely low, indicating the low predictive power of the current risk stratification criteria.
Keywords
Hypertrophic cardiomyopathy (HCM) is a genetic disease caused by mutations in various genes encoding sarcomeric proteins. The condition is characterized by unexplained left ventricular hypertrophy and shows considerable heterogeneity in its clinical manifestations.1 The most serious complication is sudden death, which is particularly frequent and shocking in young patients. The incidence of sudden death in unselected populations is lower than that found in initial series from referral centers and is currently estimated to be less than 1% per year.2–4 Most sudden deaths in this population are due to ventricular fibrillation. Because pharmacological therapy has failed to show a protective effect,5 an implantable cardioverter-defibrillator (ICD) is the only effective treatment for reducing the risk of sudden death, although no randomized studies have been performed in patients with HCM.6 An ICD is indicated for secondary prevention in high-risk patients, defined as those who have already experienced episodes of sustained ventricular tachycardia or ventricular fibrillation.7 In the remaining patients, ICD implantation is based on an individualized estimation of the risk of sudden death. In an attempt to identify high-risk patients, the following 5 clinical criteria derived from cohort studies are considered major risk factors8,9: the presence of unexplained syncope, a history of sudden death in first-degree relatives, ventricular wall thickness ≥ 30mm, nonsustained ventricular tachycardia on Holter monitoring, and abnormal blood pressure response (flat or hypotensive response) on exercise testing. Clinical practice guidelines of the European Society of Cardiology on HCM (2003)10 and prevention of sudden death (2006)11 and the most recent guidelines of the American College of Cardiology Foundation/American Heart Association (2011)12 recommend consideration of prophylactic ICD implantation in patients with 1 or more major risk factors, despite recognizing that most will not receive defibrillator therapies for many years. Few data are available on the follow-up of patients who receive an ICD following the indications of these guidelines.13–15 The aim of the present study was to analyze the follow-up of patients with HCM who received an ICD in the routine clinical practice setting in 3 centers normally attending patients with this disease.
METHODSThis is a retrospective observational study of all patients with HCM who received an ICD between January 1996 and December 2012 in 3 tertiary centers in the province of Barcelona: Hospital Universitari Vall d’Hebron (Barcelona), Hospital Universitari de Bellvitge (L’Hospitalet de Llobregat), and Hospital Universitari Germans Trias i Pujol (Badalona). Diagnosis of HCM was based on echocardiographic findings of a ventricular wall thickness ≥ 15mm with no identifiable cause. Implantation of an ICD was indicated for secondary prevention in patients who had experienced spontaneous sustained ventricular arrhythmias or aborted sudden death. Implantation of an ICD was indicated for primary prevention in patients who had 1 or more risk factors for sudden death. Primary consideration was given to the 5 major risk factors listed above, although other risk-modifying factors were also considered, such as age, the presence of outflow tract obstruction, and the presence of significant fibrosis on magnetic resonance imaging. Informed consent was obtained from all patients before defibrillator implantation.
Clinical follow-up and ICD interrogation were performed at least every 6 months. Devices were programmed according to the criteria of the attending physician. Device electrograms were analyzed to classify therapies as appropriate or inappropriate. Appropriate therapies encompassed high-energy discharges or antitachycardia therapies administered in response to sustained ventricular tachycardia. Inappropriate therapies encompassed high-energy discharges administered in the absence of ventricular arrhythmia.
The main outcome variable was appropriate ICD therapy-free survival. The incidences of inappropriate discharges and ICD-related complications were also analyzed, as well as overall mortality.
Statistical AnalysisStatistical analysis was performed with SPSS version 17.0 statistical software. Continuous variables are presented as mean (standard deviation), whereas categorical variables are presented as percentages. The duration of follow-up was calculated from the time of implantation until the last follow-up (June 2013 or date of death). Between-group comparisons were performed with a chi-square test for categorical variables and a Mann-Whitney U test for quantitative variables. The incidence of appropriate therapies in the primary and secondary prevention groups was calculated per every 100 patient-years, considered the time from implantation until the first appropriate therapy, and cumulative rates were estimated using the Kaplan-Meyer method. Between-group comparisons were performed using a log rank test. Associations between various clinical variables and the incidence of appropriate therapies were analyzed using Cox regression analysis, and the results are expressed as hazard ratios with the corresponding 95% confidence intervals. P<.05 was considered significant.
RESULTSThis study included 69 patients, of which 55 (79.3%) were men, with a mean (standard deviation) age at device implantation of 44.8 (17) years (interval, 13–73 years). An ICD was indicated for secondary prevention in 21 patients (30.4%) and for primary prevention in 48 (69.6%). Of the 21 patients in secondary prevention, 13 had experienced aborted sudden death due to ventricular fibrillation and 8 had had sustained ventricular tachycardia. From 1996 until 2003, 9 defibrillators were implanted, 6 for secondary prevention. From 2004, the number of implanted devices significantly increased, mainly in primary prevention: 18 between 2004 and 2008 (72% in primary prevention) and 42 between 2009 and 2012 (76% in primary prevention).
Of the 48 patients in primary prevention, most (64.7%) had 2 or more major risk factors and 14 (29.2%) had only 1 risk factor: 6 with syncope, 4 with a family history of sudden death, 3 with hypertrophy > 30mm, and 1 with nonsustained ventricular tachycardia on Holter monitoring. An ICD was implanted in 3 patients in primary prevention (6.3%) even though they had no classical risk factors: one was referred from another center where ventricular fibrillation was induced in an electrophysiology study; another had shown massive fibrosis on magnetic resonance imaging; and the last was a young patient with a significant outflow tract gradient, with refractory and limiting symptoms, who received a defibrillator implant for sequential dual-chamber pacing. Outflow tract obstruction, considered a risk-modifying factor, was found in 28 patients (58.3%).
Of the 21 patients in secondary prevention, 12 (57.1%) had no previously known risk factors, 8 (38.1%) had 1 risk factor, and 1 (4.2%) had 2 risk factors; 9 of the 21 patients had outflow tract obstruction (42.9%). The mean number of risk factors per patient was 1.8 in the primary prevention group and 0.5 in the secondary prevention group (P=.029).
The risk factors of patients in primary and secondary prevention are summarized in Table 1.
Clinical Characteristics and Risk Factors Prior to Implantation in Patients in Primary and Secondary Prevention
| Primary prevention (n=48) | Secondary prevention (n=21) | P | |
|---|---|---|---|
| Age, mean (SD), y | 44.4 (15.6) | 45.6 (20.1) | .624 |
| Men, % | 77.1 | 85.7 | .526 |
| Obstructive disease (gradient > 30 mmHg) | 28 (58.3) | 9 (42.9) | .235 |
| Risk factors | |||
| Family history of sudden death | 20 (41.7) | 1 (4.8) | .002 |
| Syncope | 25 (52.1) | 4 (19.0) | .010 |
| NSVT on Holter | 16 (33.3) | 3 (14.3) | .103 |
| Thickness > 30 mm | 17 (35.4) | 2 (9.5) | .027 |
| Abnormal BP response | 10 (20.8) | 0 (0.0) | .001 |
| Number of risk factors (mean) | 1.8 | 0.5 | .029 |
BP, blood pressure; NSVT, nonsustained ventricular tachycardia; SD, standard deviation.
Unless otherwise indicated, values are expressed as mean (standard deviation) or No (%).
Pharmacological treatment before implantation consisted of beta-blockers in 37 patients (53.6%), amiodarone in 6 (8.7%), and calcium antagonists in 2 (2.9%).
Most defibrillators (50 patients, 72.5%) were dual-chamber devices, although only 19 (27.5%) required pacing to reduce the pressure gradient or, less frequently, to treat sinus bradycardia.
The median follow-up duration was 40.5 months. Although there were more patients in secondary prevention in the first few years of the study, there was no significant difference in follow-up length between the primary and secondary prevention groups. No patients were lost to follow-up.
Appropriate TherapiesOf the 21 patients in secondary prevention, 14 (66.7%) had appropriate therapies during follow-up. Most (11 patients) received ICD discharges, and 3 patients received only 1 antitachycardia therapy each.
Of the 48 patients in primary prevention, 3 (6.3%) had appropriate therapies. Of these, 1 patient received antitachycardia therapy and discharges. The other 2 patients received no discharges, only antitachycardia therapy. The only patient in primary prevention who received high-energy ICD discharges was older (73 years) and had severe outflow tract obstruction (120 mmHg), functional class III, and recurrent syncope, as well as a family history of sudden death. Although no Holter monitoring was performed, sustained ventricular tachycardia was induced in an electrophysiological study. This patient with advanced disease had a first appropriate therapy only 2 months after ICD implantation and died 3 years later of heart failure.
The overall incidence of appropriate therapies was 7.8/100 patient-years, 32.7/100 patient-years in the secondary prevention group and 1.7/100 patient-years in the primary prevention group (P<.001). Most appropriate therapies (58.8%) occurred in the first year after implantation (range, 4 days-7 years). Appropriate therapies in the 3 patients in primary prevention occurred after a mean of 40.5 months following implantation, whereas the mean time to therapy was 18.3 months for patients in secondary prevention (not significantly different).
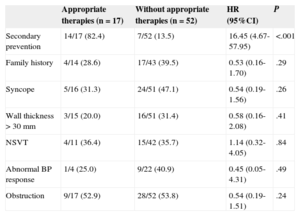
In univariate Cox regression analysis, the only factor associated with the presence of appropriate therapies was a history of sustained ventricular arrhythmias before implantation (ie, implantation for secondary prevention) (Table 2). None of the classical risk factors by themselves predicted appropriate ICD therapy. Of the 17 patients who received appropriate therapies (including both primary and secondary prevention patients), 5 had no known previous risk factors, 9 had one risk factor, and 3 had 2 or more risk factors.
Association Between Risk Factors and the Incidence of Appropriate Therapies. Univariate Analysis (Cox Regression)
| Appropriate therapies (n=17) | Without appropriate therapies (n=52) | HR (95%CI) | P | |
|---|---|---|---|---|
| Secondary prevention | 14/17 (82.4) | 7/52 (13.5) | 16.45 (4.67-57.95) | <.001 |
| Family history | 4/14 (28.6) | 17/43 (39.5) | 0.53 (0.16-1.70) | .29 |
| Syncope | 5/16 (31.3) | 24/51 (47.1) | 0.54 (0.19-1.56) | .26 |
| Wall thickness > 30 mm | 3/15 (20.0) | 16/51 (31.4) | 0.58 (0.16-2.08) | .41 |
| NSVT | 4/11 (36.4) | 15/42 (35.7) | 1.14 (0.32-4.05) | .84 |
| Abnormal BP response | 1/4 (25.0) | 9/22 (40.9) | 0.45 (0.05-4.31) | .49 |
| Obstruction | 9/17 (52.9) | 28/52 (53.8) | 0.54 (0.19-1.51) | .24 |
95%CI, 95% confidence interval; BP, blood pressure; HR, hazard ratio; NSVT, nonsustained ventricular tachycardia.
Values express no./No. (%).
During follow-up, 7 patients (10.1%) died, 3 in the primary prevention group (6.2% mortality) and 4 in the secondary prevention group (19.0% mortality). There were 5 deaths due to cardiac causes (2 due to heart failure, 2 due to sudden death without arrhythmias recorded by the ICD, and 1 due to arrhythmic storm), 1 patient died of stroke, and 1 patient died of respiratory septic shock.
Implant Complications and Inappropriate DischargesImplant-related complications were encountered in 6 patients (8.7%), 4 in the primary prevention group and 2 in the secondary prevention group. The complications were 2 pneumothorax, 2 device infections, and 2 lead malfunctions requiring reintervention.
During follow-up, 9 patients (13%) received inappropriate discharges, 4 in primary prevention and 5 in secondary prevention. These inappropriate discharges were due to supraventricular arrhythmias in 7 patients (atrial fibrillation in 5 patients, paroxysmal supraventricular tachycardia in 2) and due to signal oversensing in 2. There were no significant differences between the primary and secondary prevention groups in the rates of complications or inappropriate discharges.
DISCUSSIONThe following conclusions can be drawn from this series of unselected patients from 3 centers whose ICD was implanted according to clinical practice guidelines: although patients in secondary prevention are a high-risk group with elevated rates of recurrence and appropriate ICD therapies, there were only 3 appropriate therapies in the 48 patients in primary prevention, with a mean follow-up of 45 months. Moreover, only 1 of these patients received high-energy discharges, with the other 2 only receiving antitachycardia therapy. Thus, the usefulness of these therapies is debatable. Appropriate therapies do not always indicate a life saved,16 because patients with HCM frequently have nonsustained ventricular tachycardia that is often asymptomatic.17 These nonsustained ventricular tachycardias can be detected by the ICD, triggering an antitachycardia therapy that could be avoided by, for example, lengthening the detection intervals to allow the tachycardia to self-limit.18 Device programming is not systematically performed, allowing certain appropriate therapies to be avoided.
Accordingly, and because the annual incidence of sudden death in the HCM population is approximately 1%, the similar rates of appropriate therapies in the patients with an indication for an ICD and of sudden death observed in unselected patients suggest that the current selection criteria may not enable reliable prediction of the occurrence of sudden death or ventricular arrhythmias. Proof of this low predictive power is that more than half of the patients included in secondary prevention because of sustained ventricular arrhythmias or sudden death did not present a priori any of the criteria that would have indicated ICD implantation for primary prevention. The use of new techniques such as genetic analysis19,20 and fibrosis quantification with magnetic resonance imaging21 could improve the selection of candidates for ICD implantation in primary prevention.
Another relevant aspect concerns the ICD complications, which were not insignificant in this series. Notably, the rate of complications (including inappropriate discharges) was more than double that of appropriate therapies in the primary prevention group. Patients with HCM are more likely to experience complications and inappropriate discharges due to their younger age and higher prevalence of atrial fibrillation.22 Moreover, these young patients would need multiple reinterventions due to battery depletion and possible lead malfunction over time, increasing the long-term complication rate.23
Two patients experienced sudden death without arrhythmia recognition by the ICD. Therefore, patients with HCM can experience sudden death from nonarrhythmic mechanisms, with the ICD implant failing to guarantee absolute protection.
LimitationsAmong the limitations of the present study are the small sample size and its retrospective design. The 3 participating centers have specific databases for patients with ICDs, so patient inclusion and device follow-up were exhaustive. However, no analysis was performed of the data from of patients with HCM who did not receive an ICD, because they had no indication or for other reasons and so there may be a selection bias. In the 3 centers, the decision to implant an ICD was reached by consensus among distinct members of the medical team, including clinical cardiologists and rhythmologists, taking into account the individual risk estimated for each patient and the recommendations of the clinical practice guidelines. In addition, the cohort of patients with ICD in primary prevention would be formed by those individuals with a predicted elevated risk of sudden death, which, if there were a selection bias, would overestimate the benefit of an ICD.
The follow-up time was longer for patients in secondary prevention, possibly increasing the percentage of patients with delayed appropriate therapies. This difference in the mean follow-up time was not significant and, because most therapies occurred in the first few years of follow-up, it would be unlikely to affect the results obtained.
Despite these limitations, we consider this study to be representative of the results expected for most patients with HCM in real clinical practice in nonreferral centers for this disease.
CONCLUSIONSPatients who received an ICD implantation as primary prophylaxis of sudden death following the current clinical practice guidelines have an excessively low rate of appropriate therapies that is similar to the rate of sudden death observed in unselected patients with HCM, indicating that the current risk stratification criteria have a low predictive power and should be revised. Patients who have had sustained arrhythmias are a high-risk group and ICD implantation is clearly justified for them.
CONFLICTS OF INTERESTSNone declared.