A standardized protocol of emergent transfer for primary percutaneous coronary intervention for patients with ST elevation myocardial infarction, defined as the Infarction Code, was implemented in June 2009 in the Catalan regional health system. The objective of this study was to evaluate the impact of the new protocol on delay times, number of procedures and clinical characteristics compared with the previous period in the population of patients referred to our hospital.
MethodsAll consecutive patients undergoing primary percutaneous coronary intervention in our hospital were prospectively registered. The clinical characteristics, delay times and mortality in the follow-up of the protocol implementation period (June 2009-May 2010) were analyzed and compared with the previous year (June 2008-May 2009).
ResultsDuring the protocol period, 514 patients were included, compared with 241 in the previous year. Age, cardiovascular risk factors, anterior myocardial infarction and procedure characteristics were similar in the 2 groups. The first medical contact to balloon time was lower in the protocol period (median time 120min vs 88min; P<.001). Patients in the protocol period showed a trend toward less severe disease (Killip III, rescue angioplasty). The multivariate regression analysis showed a significant association between 1-year mortality and age, Killip class≥III at admission, anterior infarction and 3-vessel disease.
ConclusionsThe introduction of the Infarction Code program increased the number of patients treated by primary percutaneous coronary intervention with a reduction in delay times and better clinical characteristics at presentation.
Keywords
.
IntroductionPrimary angioplasty (PA) is the treatment of choice in acute ST-segment elevation myocardial infarction (STEMI).1, 2, 3 This recommendation is based on ensuring that the time between first seeking medical attention and opening the artery is less than 120min; the second-line option is fibrinolytic treatment.4 The benefit of PA with respect to fibrinolysis lies, in addition to a greater intrinsic efficacy of the procedure in reestablishing flow to the artery, in the speed with which it is performed.5, 6 Thus, the delay from the time the patient arrives in the emergency room to opening of the artery is a crucial factor in determining the efficacy of the procedure and the results.7 Several registries have shown that short delay times are associated with better outcomes in terms of mortality.8, 9 Thus, systematic performance of PA should be accompanied by a system that guarantees rapid diagnosis and immediate transfer to carry out the PA. In Spain and other European countries, there are several examples of care programs for STEMI, based on the integration of a central triage system and a network of referral hospitals that offer reperfusion treatment and district hospitals according to a hub and spoke arrangement.10, 11 Such an approach has allowed more widespread use of reperfusion in the acute phase of infarction and has had a clear impact on clinical outcomes.12, 13
On June 1, 2009, an integrated care network, known as the Infarction Code was implemented in Catalonia for systematic performance of PA. The objective of this study was to detect changes resulting from the implementation of the Infarction Code among patients with STEMI treated with angioplasty in the first 12h of infarction in terms of the number of patients attended, delay times, and clinical profile on arrival. The 1-year outcomes were also analyzed.
MethodsThis retrospective study was performed using a prospective registry that included all consecutive patients referred to our center for reperfusion by PA or rescue angioplasty, with a diagnosis of STEMI: typical chest pain and persistent ST elevation or new onset left bundle branch block.2
A single database was designed to collect the baseline data and data on the procedure in the catheterization laboratory. The clinical outcome in the coronary unit was then recorded.
This study comprised 2 periods of 1 year each: PreCode phase (June 1, 2008 to May 31, 2009), and the Infarction Code phase (June 1, 2009 to May 31, 2010).
PreCode PhaseDuring this period, percutaneous reperfusion treatment by means of PA was performed on:
• All patients who attended the emergency room of our hospital.
• Patients transferred by the emergency medical services (EMS) from home or from primary care centers.
• Patients with contraindications for thrombolysis, referred from other hospitals.
Patients treated with thrombolysis in other centers who did not show signs of reperfusion 90min after administration of fibrinolytic treatment were transferred to our center for rescue angioplasty. In all cases, the patient was assessed directly by the cardiology team on call in our hospital. The team decided on the indication for reperfusion and contacted the interventional cardiology unit to perform the procedure. In the PreCode phase, the patients’ referral area was the same as in the Infarction Code phase. The difference was that there was no protocol for STEMI: there was no standard strategy for prioritizing patient transfer or systematic prior contact with the EMS and the duty cardiologist.
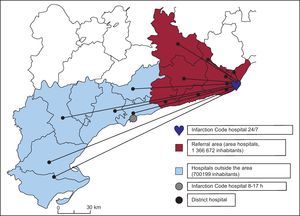
Infarction Code ProcedureThe Infarction Code was implemented in Catalonia on June 1, 2009, in order to offer PA treatment to all patients diagnosed with STEMI and to guarantee shorter times between seeking medical assistance and opening of the artery. The autonomous community was divided into referral areas according to territorial sectors. The referral area of our hospital (Figure 1)14 includes 6 districts in the provinces of Barcelona and Tarragona,14 which include 8 hospitals (area hospitals). Outside office hours (Monday to Friday from 17:00, and Saturdays and holidays), our hospital is also the referral hospital for other districts in the province of Tarragona,14 which includes 7 hospitals (outside-area hospitals). When a healthcare team able to refer a patient for reperfusion therapy (primary healthcare centers, EMS, hospitals) diagnoses STEMI, they contact the EMS. When diagnosis is confirmed and transfer is decided, the Infarction Code is considered activated. The EMS contacts the duty cardiology team (single direct line) to report the patient's arrival, and the duty cardiologist directly alerts the interventional cardiology unit in the catheterization laboratory while the patient is in transfer. At the time of activation, double antiplatelet therapy is administered (acetylsalicylic acid and clopidogrel 600mg) along with anticoagulation therapy (unfractionated heparin, 1mg/kg as an intravenous bolus).
Figure 1. Referral area of our center. Hospitals inside and outside the area.
With patients who report directly to the emergency room of our hospital, the operation of the Infarction Code is no different to the previous period: a call is made to the duty cardiologist, who alerts the interventional cardiology unit.
In patients who need a transfer from other hospitals to perform PA, if the coordinating center of the EMS considers that the expected time between diagnosis and opening of the artery would clearly exceed 120min, in situ fibrinolytic treatment is recommended; if this is ineffective, the patient is transferred for rescue angioplasty.
The Infarction Code was implemented at the extrahospital level by reorganizing ambulance flows, without increasing the resource needs of the transport system. Within the hospital, the increase in resources took the form of the availability of 2 intermediate care beds after interventions and hiring an additional nurse and a part-time interventional cardiologist.
Given the increase in the volume of work associated with implementing the program, and in order to offer appropriate care to all patients without overloading the interventional center, a protocol for return to the referring hospitals was drawn up. This protocol stipulates that after the procedure, hemodynamically stable patients with no complications are transferred to their referring hospital. Patients can be transferred to a hospital with an intensive care unit 8h after reperfusion and can return to a hospital with conventional wards after 24h have elapsed.
Study VariablesData on classification, origin, clinical characteristics, infarction, and procedure were collected.
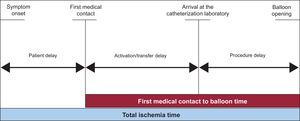
For times, the following parameters were collected: time of symptom onset, time of first medical contact (time in which the first diagnostic electrocardiogram was performed and percutaneous reperfusion treatment was indicated),15 time of arrival in the catheterization laboratory, and time of opening of the artery (time when the first device is introduced).
From these times, the following delays were derived (Figure 2): patient delay (time from the onset of symptoms to the first medical contact), delay in activation/transfer (time from first medical contact to arrival in the catheterization laboratory), and procedure delay (time from arrival in the catheterization laboratory until artery opening).
Figure 2. Periods of time analyzed.
Finally, the following intervals were calculated: time from first medical contact to reperfusion (FMCtoB, time from first medical contact to balloon), and total ischemia time (time from symptom onset until opening of the artery).
The success of the reperfusion procedure was defined as final TIMI (Thrombolysis in Myocardial Infarction) flow grade 3 and residual stenosis<20%.16
Clinical follow-up at 30 days and 1 year was performed. Data on mortality (overall and cardiovascular related), reinfarction, repeat revascularization of the “culprit” vessel, and stroke were collected. The composite outcome of all-cause mortality, reinfarction, and repeat revascularization of the culprit lesion at 30 days and 1 year was analyzed.
Statistical AnalysisQuantitative variables are shown as means (standard deviation) and were compared using the Student t test; if they did not follow a normal distribution, they were reported as medians [interquartile range] and compared using the Mann-Whitney rank test. Categorical variables were compared using the Pearson χ2 test and described using absolute numbers and percentages.
The survival analysis was performed using the Cox proportional risks analysis with stepwise regression. The multivariate analysis included variables that were significant in the univariate comparison (P<.1) and clinically relevant variables according to previous publications. The assumption of proportionality was checked by introducing interactions between the study variables and survival time into the model. Survival curves were constructed from the Cox model. Significance was set at a bilateral P value<.05. SPSS (version 18.3) software was used for the statistical analysis (SPSS Inc., Chicago, Illinois, United States).
Results Clinical CharacteristicsIn the PreCode phase (June 1, 2008 to May 31, 2009), percutaneous reperfusion was performed in our center in the first 12h of infarction in 241 patients, whereas during the Infarction Code phase (June 1, 2009, to May 31, 2010), 514 reperfusion procedures were performed, representing an increase of 112%.
During the Infarction Code period, 566 emerging catheterization procedures were performed (PA indication or rescue angioplasty), of which 52 (9.2%) were considered false alarms, and were not included in the analysis. The patients’ clinical characteristics are summarized in Table 1; no significant differences between groups were found. A significant increase in the percentage of PA compared to rescue angioplasty was observed. An increase in the rate of use of bivalirudin as an anticoagulant was identified in the Infarction Code phase.
Table 1. Baseline Characteristics of the Event and the Procedure.
| PreCode (n=241) | Infarction Code (n=514) | P | |
| Demographic Data | |||
| Age | 61.5±12.6 | 61.8±13.4 | .75 |
| Women | 44 (18.3) | 103 (20) | .6 |
| History | |||
| Smoker | 119 (49.4) | 228 (44.4) | .2 |
| Dyslipidemia | 138 (57.3) | 266 (51.8) | .16 |
| Hypertension | 138 (57.3) | 294 (57.2) | .99 |
| Diabetes mellitus | 57 (23.7) | 137 (26.7) | .4 |
| Renal failure | 11 (4.6) | 31 (6) | .4 |
| Peripheral vascular disease | 26 (10.8) | 32 (6.2) | .03 |
| Prior AMI | 26 (10.8) | 63 (12.3) | .6 |
| Prior PCI | 18 (7.5) | 39 (7.6) | .95 |
| Prior coronary artery surgery | 6 (2.5) | 7 (1.4) | .21 |
| Infarction data | |||
| Anterior AMI | 100 (41.5) | 238 (46.3) | .22 |
| Inferior AMI | 133 (55) | 252 (49) | .12 |
| Killip≥III | 24 (10) | 31 (6) | .081 |
| Cardiogenic shock | 11 (4.6) | 17 (3.3) | .4 |
| OTI | 17 (7.1) | 23 (4.5) | .14 |
| Treatment | |||
| ASA | 238 (98.7) | 507 (98.6) | .5 |
| Clopidogrel | 229 (95) | 488 (94.9) | .99 |
| Heparin | 230 (95) | 492 (96) | .98 |
| Abciximab | 45 (18.7) | 109 (21.2) | .44 |
| Bivalirudin | 31 (12.9) | 125 (24.3) | <.001 |
| Data on the Procedure | |||
| Primary angioplasty | 195 (80.9) | 486 (94.6) | <.001 |
| Rescue angioplasty | 46 (19.1) | 28 (5.4) | <.001 |
| Out of office hours | 113 (47) | 286 (64) | <.001 |
| IAoBCP | 13 (5.4) | 9 (1.8) | .009 |
| Initial TIMI flow 0-1 | 177 (73.6) | 396 (77.4) | .32 |
| Three-vessel disease | 43 (17.8) | 97 (18.9) | .77 |
| Thrombus aspiration | 167 (69) | 365 (71) | .67 |
| Coronary stenting | 223 (92.5) | 476 (92.6) | .97 |
| Final TIMI flow 3 | 229 (95) | 488 (94.9) | .99 |
| Angiographic success | 227 (94.2) | 480 (93.2) | .75 |
| Zwolle score | 3.6 [1-4] | 3.2 [1-4] | .12 |
AMI, acute myocardial infarction; ASA, acetylsalicylic acid; AVB, atrioventricular block; IAoBCP, intraaortic balloon counter pulsation; OTI, orotracheal intubation; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
The results are expressed as mean±standard deviation, no. (%), or median [interquartile range].
No significant differences were observed for successful outcome of the procedure. Of note was a greater trend toward greater severity in the PreCode phase. The variables of hypotension, cardiogenic shock, advanced Killip class, and intraaortic balloon counter pulsation occurred more frequently in the PreCode phase.
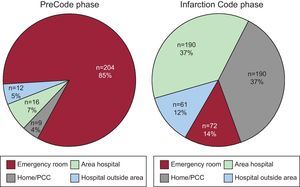
Patient Origin and Site of Code ActivationDuring the Infarction Code phase, the number of patients transferred by EMS from home or the primary care center (116 vs 190) and from hospitals in the referral area (35 vs 191) increased, whereas the number of patients who spontaneously attended the emergency room in our center remained the same (Table 2). Overall, the number of patients referred from a noninterventional center increased from 24% (n=57) to 49% (n=252). The decision to activate the PA system in the PreCode period was taken almost exclusively in our hospital on the arrival of the patient. In contrast, after implementing the Infarction Code, 86% of the activations occurred outside our center (Figure 3). As a result of this modification, the percentage of patients who entered the catheterization laboratory directly without referral from the emergency room was 11% (n=26) in the PreCode phase and 67% (n=327) in the Infarction Code phase. The number of procedures performed outside office hours significantly increased (Table 1).
Table 2. Delay Between First Medical Contact and Reperfusion According to Period, Origin, and Indication for Reperfusion.
| PreCode | Infarction Code | P | |
| A. Overall population | |||
| Home/PCC (n=116/190) | 125 [90-160] | 80 [63-105] | <.001 |
| Emergency room (n=68/72) | 82 [60-125] | 70 [50-84) | .03 |
| In-area hospital (n=35/191) | 135 [106-200] | 90 [75-110] | <.001 |
| Outside-area hospital (n=22/61) | 218 [168-300] | 132 [105-155] | <.001 |
| B. Only primary angioplasty | |||
| Home/PCC (n=104/189) | 123 [89-160] | 80 [63-105] | <.001 |
| Emergency room (n=68/72) | 82 [60-125] | 70 [50-84] | .03 |
| In-area hospital (n=16/191) | 130 [105-165] | 90 [75-110] | .001 |
| Outside-area hospital (n=7/34) | 215 [160-235] | 119 [95-148] | <.001 |
PCC, primary care center.
Times (minutes) as median [interquartile range].
Figure 3. Site of indication of reperfusion. Differences between PreCode and Infarction Code phases. PCC, primary care center.
Delay TimesTable 3 details the changes observed in delay times. A significant reduction in the median total ischemia time of 30min (12.2% reduction) was observed. A more marked median reduction in FMCtoB of 32min (26.7% reduction) was also observed. In the detailed analysis of the delays, a decrease in the activation/transfer time of 29min was observed, whereas the in-hospital delay was reduced by a median of 3min. No significant changes in patient delay were observed.
Table 3. Delay Times.
| PreCode | Infarction Code | P | |
| A. Overall population, no. | 241 | 514 | |
| FMCtoB | 120 [85-165] | 88 [68-114] | <.001 |
| Total ischemia time | 246 [180-390] | 216 [166-330] | .001 |
| Patient delay | 110 [75-215] | 115[60-184] | .2 |
| Activation/transfer delay | 85 [53-130] | 56 [40-85] | <.001 |
| In-hospital delay | 30 [25-40] | 27 [20-35] | <.001 |
| B. Primary angioplasty, no. | 195 | 482 | |
| FMCtoB | 115 [80-160] | 85 [66-110] | <.001 |
| Total ischemia time | 225 [170-360] | 210 [165-310] | .02 |
| Patient delay | 107 [70-203] | 111 [57-175] | .3 |
| Activation/transfer delay | 80 [45-120] | 55 [39-80] | <.001 |
| In-hospital delay | 30 [25-40] | 27 [20-35] | <.001 |
FMCtoB, first medical contact to balloon time.
Times (minutes) as median [interquartile range].
The percentage of patients with FMCtoB<120min was significantly higher in the Infarction Code group (81% vs 51%; P<.001).
Given the greater percentage of patients treated with rescue angioplasty in the PreCode phase, the delay times in the subgroup of patients treated with PA were analyzed, and the same differences as those observed for the overall population were maintained (Table 3B).
Analysis according to referral site showed a decrease in delay times for all sites where the Infarction Code was activated (Table 2). This result was maintained when only patients who underwent PA were analyzed, and a significant decrease in FMCtoB was observed, even in the cases of transfers over larger distances (from outside-area hospitals).
Clinical Outcomes MortalityThe overall mortality of the patients with STEMI treated by angioplasty in the first 12h of the infarction was 6.5% at 30 days and 9.8% at 1 year of follow-up. The crude all-cause mortality rates at 30 days and 1 year were lower in the Infarction Code group (Table 4A). Table 4B expresses the data pertaining to patients who underwent PA and shows similar results to those of the overall population.
Table 4. Descriptive Analysis of Clinical Outcomes.
| PreCode | Infarction Code | P | |
| A. Overall population | 241 | 514 | |
| Events at 30 days | |||
| Total mortality | 22 (9.1) | 27 (5.3) | .044 |
| Cardiac mortality | 21 (8.7) | 23 (4.5) | .02 |
| Reinfarction | 5 (2.1) | 7 (1.4) | .54 |
| RRCV | 4 (1.7) | 6 (1.2) | .74 |
| IS | 3 (1.2) | 3 (0.6) | .39 |
| MCVE | 26 (10.8) | 34 (6.8) | .063 |
| Events at 1 year | |||
| Total mortality | 33 (13.7) | 41 (8) | .018 |
| Cardiac mortality | 27 (11.2) | 30 (5.8) | .012 |
| Reinfarction | 13 (5.4) | 13 (2.6) | .06 |
| RRCV | 11 (4.6) | 16 (3.2) | .4 |
| MCVE | 45 (18.8) | 57 (11.5) | .007 |
| B. Primary angioplasty | 195 | 486 | |
| Events at 30 days | |||
| Total mortality | 15 (7.7) | 25 (5.1) | .21 |
| Cardiac mortality | 14 (7.2) | 21 (4.3) | .13 |
| Reinfarction | 5 (2.6) | 6 (1.3) | .31 |
| RRCV | 4 (2.1) | 5 (1.1) | .46 |
| IS | 1 (0.5) | 2 (0.4) | 1 |
| MCVE | 19 (9.7) | 31 (6.6) | .19 |
| Events at 1 year | |||
| Total mortality | 26 (13.3) | 37 (7.6) | .016 |
| Cardiac mortality | 20 (10.3) | 26 (5.3) | .027 |
| Reinfarction | 10 (5.1) | 11 (2.3) | .08 |
| RRCV | 8 (4.1) | 15 (3.2) | .6 |
| MCVE | 34 (17.4) | 52 (10.9) | .03 |
IS, ischemic stroke; MCVE, major cardiovascular events (death, reinfarction, or repeat revascularization); RRCV, repeat revascularization of “culprit” vessel.
Data are presented as no. (%).
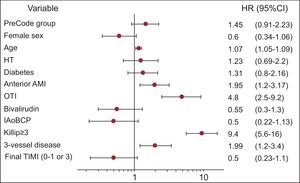
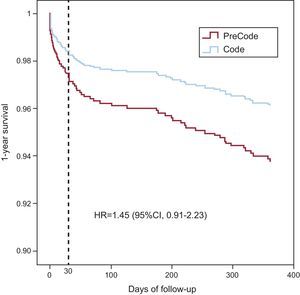
The multivariate analysis (Figure 4) showed that the variables predictive of mortality at 1 year were age, anterior infarction site, Killip class≥III on arrival at the catheterization laboratory, and multivessel disease. The variable of study period showed a trend to lower mortality in the Infarction Code group, although the differences were not statistically significant. The survival curves constructed from the multivariate analysis are shown in Figure 5. The multivariate analysis limited to patients who underwent PA showed that the variables predictive of 1-year mortality were the same as those for the overall population and confirmed that the variable of study period was associated with lower mortality in the Infarction Code group, although the differences were not statistically significant (hazard ratio=1.67; 95% confidence interval, 0.98-2.9; P=.06).
Figure 4. Multivariate analysis of predictors for mortality at 1 year of follow-up. 95%CI, 95% confidence interval; AMI, acute myocardial infarction; HR, hazard ratio; HT, hypertension; IAoBCP, intraaortic balloon counter pulsation; OTI, orotracheal intubation; TIMI, Thrombolysis in Myocardial Infarction.
Figure 5. Survival curves at 1 year. 95%CI, 95% confidence interval; HR, hazard ratio.
Reinfarction, Revascularization, StrokeThe reinfarction rates at 30 days and 1 year showed no significant differences between the 2 groups; a greater trend toward a higher incidence of reinfarction at 1 year of follow-up was observed in the PreCode group.
The revascularization rate of “culprit” lesions at 30 days and 1 year showed no significant differences.
The stroke rates at 30 days were low in both groups. Although the incidence was 2-fold higher in the PreCode group, this difference was not statistically significant.
Analysis of the composite endpoint of death, reinfarction, and new revascularization showed a significant difference between the 2 groups at 1 year of follow-up (Table 4); this difference was not maintained in the multivariate analysis of survival.
DiscussionImplementation of the Infarction Code has radically changed the care of patients with STEMI in our population. In line with previously studies, we observed an almost 2-fold increase in the number of patients who received percutaneous reperfusion treatment in the acute phase of acute myocardial infarction and a significant decrease in rescue angioplasty13 in our catheterization laboratory.
Delay TimesThe present study showed a clear improvement in delay times compared with the period prior to the Infarction Code in patients treated with angioplasty in the first 12h. Currently, the vast majority of patients have FMCtoB that meet the recommendations of the European Guidelines.2 Among the modifications provided by the new protocol, we wish to emphasize the effectiveness of extrahospital activation of PA, which, along with direct transfer to the catheterization laboratory, has been shown to have a significant impact on reducing delay times.17 We should highlight that in our study, we refer to the time of first medical contact as the time in which reperfusion treatment was indicated, whether in our center, extrahospital care, or in another hospital unable to perform PA. This distinction is important because some recent publications on delay times in the United States6, 18 exclude patients transferred from other centers (up to a third of the total), and the delay was calculated relative to the arrival of the patient at the hospital with PA facilities. Measurement of the times from therapeutic indication seems to be a more appropriate strategy, as it allows the overall functioning of the health system to be assessed.15 Our results are comparable to those published in this journal by Rodríguez-Leor et al.,19 who reported very similar total ischemia and FMCtoB times. In our series, compared with that study, the patients transferred from other centers, despite a larger and more populated referral area, showed shorter delay times, in line with those recommended in the guidelines. Implementation of the Infarction Code has allowed a greater reduction in FMCtoB compared with the previous period in this group in particular.
With regard to patients from centers outside the referral area, despite a decrease in times compared with the PreCode phase, FMCtoB still has values above those recommended by the guidelines; further efforts are needed to optimize the transfer strategy and comply with recommendations in order to maintain the clinical benefit of PA and guarantee homogenous care for the entire population.
Nevertheless, given that some transfers are up to 200km, the times obtained do not differ from those of other registries in the “real world” that have assessed similar situations.8, 10, 20
Clinical EventsThe mortality results were similar to those obtained in care programs for STEMI at a national level21 and were also in line with data published from international trials and clinical registries.9, 22, 23, 24 Of note is the tendency to improved clinical profile on admission, with a lower incidence of severe heart failure among patients of the Infarction Code group. This difference in fundamental clinical variables that impact on prognosis has several explanations. Firstly, it could be explained, in part, by a decrease in the percentage of patients undergoing rescue angioplasty, a subgroup with a worse severity profile and a tendency for poorer outcomes.25, 26 Secondly, the decrease in delay times has helped improve the severity profile on arrival in the catheterization laboratory. Finally, we cannot rule out a selection bias in our study during the PreCode period, which may have had an impact on clinical outcomes.
In comparison with other series published, we found no differences in factors predictive of mortality in our population.6, 21, 27 A larger number of patients are required to determine whether there is a significant impact on mortality on implementation of the Infarction Code. However, the reductions observed in terms of delay times and more favorable initial clinical profile on arrival at the catheterization laboratory undoubtedly had an impact on the trends observed.
The differences in mortality and clinical events observed in the 2 groups were maintained in the subgroup of patients who underwent PA. This observation underlines the importance of reducing delays after activating the Infarction Code.
An aspect of note is that, given the characteristics of our referral area, the population attended by our hospital has little intersectorial mobility, and therefore the Infarction Code program can serve to identify the real incidence at a population level of myocardial infarction treatable by reperfusion in a referral area.
LimitationsThis was a single-center, observational study and, although data were collected prospectively, some differences might not have been detected, which might have had an impact on the findings. Furthermore, as already mentioned, although all patients referred to our hospital during the study period were included, thereby ensuring good representation of the functioning of PA in the “real world”, a selection bias in the PreCode period cannot be ruled out. Indeed, we do not know the real incidence and characteristics of the infarctions treated by effective fibrinolysis or not treated with any reperfusion therapy in this period. Consequently, clinical outcomes should be interpreted with caution, although implementation of the Infarction Code is one of the largest differences between the 2 periods and is probably the main factor behind the improvements observed in the outcomes of our patients.
ConclusionsIt has been shown that implementation of the Infarction Code improves delay times for care of patients with STEMI treated in our center with PA, leading to an efficient care network, even for long-distance transfers. The effectiveness of the protocol was confirmed by the short and long-term results, with an improvement in the clinical profile of the patients on arrival at the catheterization laboratory and low mortality rates during follow-up.
FundingThis study was supported in part by an unconditional grant from Boston Scientific (P.D.D.), a grant from the Catheterization and Interventional Section of the Spanish Society of Cardiology for training in post-residence investigation, 2011 (S.H.), and an IDIBELL post-residence training program in investigation (J.G.L.). It was also conducted in part under the auspices of the Stent for Life initiative of the Spanish Society of Cardiology and the European Society for Cardiology.
Conflicts of interestNone declared.
Received 9 February 2012
Accepted 11 June 2012
Corresponding author: Unidad de Hemodinámica y Cardiología Intervencionista, Servicio de Cardiología, Área de Enfermedades del Corazón, Hospital Universitario de Bellvitge, Feixa Llarga s/n, 08007 L’Hospitalet de Llobregat, Barcelona, Spain. 26587jgh@comb.cat