Hypercoagulability and thromboembolism are processes that arise from severe acute respiratory syndrome coronavirus 2 infection and are responsible for a high degree of coronavirus disease 2019 (COVID-19)-related morbidity and mortality. This study sought to assess the effect of antiplatelet drugs on COVID-19 severity (risk of hospitalization and mortality), susceptibility to severe acute respiratory syndrome coronavirus 2 infection, and progression to severe COVID-19.
MethodsWe conducted a population-based case-control study in a northwestern region of Spain in 2020. The study involved 3060 participants with a positive polymerase chain reaction test who were hospitalized, 26 757 participants with a positive polymerase chain reaction test who were not hospitalized, and 56 785 healthy controls.
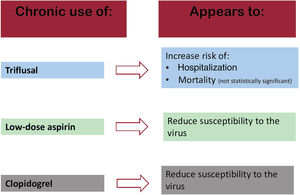
ResultsTriflusal seemed to be associated with a significant increase in risk of hospitalization (aOR, 1.97; 95%CI, 1.27-3.04) and susceptibility to infection (OR, 1.45; 95%CI, 1.07-1.96). It also appeared to lead to a nonsignificant increase in the risk of mortality (OR, 2.23; 95%CI, 0.89-5.55) and/or progression to more severe disease stages (OR, 1.42; 95%CI, 0.8-2.51). Aspirin seemed to be associated with a statistically significant decrease in susceptibility to severe acute respiratory syndrome coronavirus 2 infection (OR, 0.92; 95%CI, 0.86-0.98).
ConclusionsTriflusal use appears to increase the risk of susceptibility to COVID-19 infection and an even higher risk of hospitalization, whereas the other antiplatelets could be associated with a reduction in the risk of the various outcomes or have no effect on risk. These findings could support reconsideration of triflusal prescription in COVID-19 pandemic situations.
Keywords
Since the outbreak of the coronavirus disease 2019 (COVID-19) pandemic, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) syndrome has caused a severe health crisis resulting in 762 million cases of COVID-19 and 6.84 million deaths worldwide.1 Despite the progress made in containing the virus, the World Health Organization has warned that it still remains a public health threat.2
SARS-CoV-2 infection not only causes acute respiratory syndrome,3 but also gives rise to endothelial dysfunction and platelet and neutrophil activation, which induces a state of hypercoagulation and inflammation.4,5 Of these processes, endothelitis has proved to be the main process involved in the pathogenesis of the disease and the increase in D-dimer concentrations, which is a key indicator of severity.6 All of the above increases the risk of venous and arterial thromboebolism, which could in turn lead to multiorgan damage, increasing the risk of mortality.7–11
There is a theory that patients receiving chronic antiplatelet therapy might have a reduced reduction of COVID-19-specific thromboembolic complications. This may be due to the action of the neutrophil extracellular trap system and inhibition of the formation of microthrombi secondary to propagation of the virus.12,13 Hence, antiplatelets have been repurposed for the treatment of COVID-19, as has been done with other already existing treatments.14
A number of studies have analyzed the role of antiplatelet use in patients with COVID-19, and its related risks. However, many of them have only done so at the prehospital level, when the drug is administered in the initial phases of the disease.3,4,15,16 Some studies have analyzed the role of chronic antiplatelet uses and risk of mortality and disease severity, albeit with inconsistent results.9,17,18 Furthermore, to our knowledge, no study has simultaneously evaluated the role of chronic use of aspirin, clopidogrel and triflusal in all COVID-19 disease processes. Accordingly, the aims of this study were to assess the impact of pre-existing long-term treatment with antiplatelets (triflusal, aspirin, and clopidogrel) on severity (risk of hospitalization and mortality), susceptibility to infection, and progression to severe COVID-19.
METHODSSettingThis study was conducted in Galicia, a region in northwest Spain with a population of nearly 3 million inhabitants, almost all of whom (98%) are covered by the National Health Sytem, which is largely funded by taxation. While patients must pay between 0% and 60% of the cost of medicines, depending on their income, medical visits are free of charge. The Galician Health Service (Servicio Gallego de Salud/Servizo Galego de Saude/SERGAS), which is part of the National Health System, keeps electronic medical records on all patients. These records contains all information on the clinical care provided at the different levels of the health care process, both primary and hospital (diagnostic tests, medication prescriptions, International Classification of Primary Care codes, hospitalizations, etc).
Study design and participantsWe conducted a population-based case-control study, using an epidemiological approach.19 This design features the use of data from a representative sample of all cases (in this instance, with exhaustive sampling) in a precisely defined and identified population (ie, the population served by the Health System of Galicia), and compares them with data from individuals (controls) randomly drawn from the same population as the cases (population-based case-controls). According to Rothman, this design can be regarded as the most desirable option for a case-control study.20 The study period was March to December 2020.
To address the various objectives of the study, we conducted 4 substudies to analyze the association between chronic antiplatelet use and: a) severity of COVID-19 (risk of hospitalization and mortality); b) susceptibility to COVID-19 infection; and c) progression to severe COVID-19.
To study the severity of the disease in relation to the risk of hospitalization, cases were defined as patients admitted to any SERGAS hospital for COVID-19, with polymerase chain reaction (PCR) test confirmation, during the period between the outbreak of the pandemic and December 31, 2020, both inclusive. The time between the date of a positive PCR test and hospitalization21 was set at a maximum of 10 days, to exclude possible cases that might have been admitted for reasons other than COVID-19 infection. To improve the efficiency of our analysis of the risk of hospitalization, controls were randomly selected and matched with cases by age, sex, and primary care center. Up to 20 controls were selected for each case.
To assess disease severity in relation to mortality, a case was defined as any person who died during hospital admission with a confirmed COVID-19 positive PCR test in 2020. Controls were the same persons as those used to assess the risk of hospitalization for COVID-19.
To assess susceptibility, a case was defined as any person diagnosed with COVID-19 confirmed by a positive PCR test test (hospitalized and nonhospitalized) during the study period.21 As controls, we used the same persons as those used to assess the risk of hospitalization, characterized by the absence of a history of a positive PCR test.
To assess progression to severe COVID-19, a case was defined as any person hospitalized for COVID-19 confirmed by a positive PCR test test in 2020 (hospitalized and nonhospitalized), and controls consisted of all patients with a confirmed COVID-19 positive PCR test not hospitalized in 2020. In this model, the cases were not matched with controls.
Ethical aspectsThe study was approved by the Galician Clinical Research Ethics Comittee (Comité de Ética de Investigación de Galicia, reference 2020/349), certified by the Spanish Agency for Medicines and Medical Devices (AEMPS), and conducted in accordance with the principles of the Declaration of Helsinki and current legislation on biomedical research. The study protocol is registered in the EU Electronic Register of Post-Authorisation Studies.22 Automated data extraction was performed and anonymized to ensure that the participants concerned could not be identified and consequently it was not necessary to obtain informed consent from the participants.
Data source and collectionAll data were extracted semiautomatically by an independent information technology (IT) services company from the Complex Data-Analysis Systems (Sistemas de Información y Análisis Complejos/SIAC) used for SERGAS. The SIAC acts as a data warehouse that stores information for the management of several systems (dispensing of medicines, diagnoses and hospitalizations, among others). The datasets generated and analyzed during the current study are not publicly available due to Galician Public Health Systems Restrictions.
ExposureThe variables of exposure were antiplatelet agents (ATC code B01AC). Specifically, we included clopidogrel (B01AC04), aspirin acid (B01AC06), and triflusal (B01AC18). The use of one type of antiplatelet does not preclude the patient from using another type of antiplatelet. Those prescribed and dispensed to cases and controls alike throughout the study period were recorded, along with the use of any medication in the 6 months prior to the index date. The index date was defined as the 10th day prior to the positive PCR test date for cases and the same day as the index date of the matched cases from the hospitalization model for controls.
As study covariates, we recorded demographic and anthropometric variables, clinical COVID-19 variables (where applicable), and data on hospitalization, comorbidities (hypertension, diabetes, chronic obstructive pulmonary disease, obesity, ischemic heart disease, stroke, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker status), and exposure to the other medications (antihypertensives, diuretics, nonsteroidal anti-inflammatory drugs, hypolipidemic agents, anticoagulants, and glucocorticoids) prescribed for and dispensed to each of the participants. Polypharmacy (number of different medications prescribed and dispensed for chronic diseases in the last 6 months before the index date) was used as a proxy for the degree of chronicity in patients.23
Statistical analysisTo perform the statistical analysis, we used generalized linear mixed models24 with the binomial link function. The reason for using these models was the structure of the data and their many advantages over conditional regression.24–26 The independent variable used to construct the models was dispensed vs absence of clopidogrel, aspirin, and triflusal. To build the models, the following 4 levels were considered: patient; case-control strata (case and matched control); health center; and pandemic wave. Random effects were used to assess the effect of the pandemic wave, and nested random effects for patients, case-control strata, and health center. It was assumed that the effect of exposure on the probability of being a case (adjusted for covariates) might have been different between infection waves. Adjustments were made for potential confounding variables, including age, sex, comorbidities, smoking, and each additional pharmacological treatment. The results are expressed as adjusted odds ratios (aOR) with their 95% confidence intervals (95%CI). Adjusted estimates were obtained for the effect of dispensed antiplatelet therapy compared with the absence of any antiplatelet drug therapy. Models were estimated using the glmer function of the lme4 R package27 (R Software version 4.1.0).
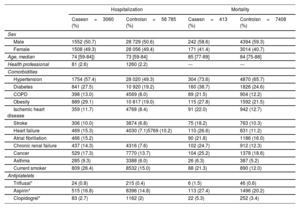
RESULTSWe collected data on 86 602 participants: of these, 3060 were cases (hospitalized participants with a positive PCR test), 413 of whom died during hospitalization, and 26 757 were nonhospitalised cases. There were 56 785 participants who did not have a positive PCR test. Among chronic users of antiplatelets, the most widely used drug was aspirin. The demographic and clinical characteristics of cases and controls can be found in table 1 and table 2.
Demographic and clinical characteristics of the cohort (severity: risk of hospitalization and mortality)
| Hospitalization | Mortality | |||
|---|---|---|---|---|
| Casesn=3060 (%) | Controlsn=56 785 (%) | Casesn=413 (%) | Controlsn=7408 (%) | |
| Sex | ||||
| Male | 1552 (50.7) | 28 729 (50.6) | 242 (58.6) | 4394 (59.3) |
| Female | 1508 (49.3) | 28 056 (49.4) | 171 (41.4) | 3014 (40.7) |
| Age, median | 74 [59-84]) | 73 [59-84] | 85 [77-89] | 84 [75-88] |
| Health professional | 81 (2.6) | 1260 (2.2) | --- | --- |
| Comorbidities | ||||
| Hypertension | 1754 (57.4) | 28 020 (49.3) | 304 (73.6) | 4870 (65.7) |
| Diabetes | 841 (27.5) | 10 920 (19.2) | 160 (38.7) | 1826 (24.6) |
| COPD | 398 (13.0) | 4569 (8.0) | 89 (21.5) | 904 (12.2) |
| Obesity | 889 (29.1) | 10 817 (19.0) | 115 (27.8) | 1592 (21.5) |
| Ischemic heart disease | 359 (11.7) | 4768 (8.4) | 91 (22.0) | 942 (12.7) |
| Stroke | 306 (10.0) | 3874 (6.8) | 75 (18.2) | 763 (10.3) |
| Heart failure | 469 (15.3) | 4030 (7.1)5769 (10.2) | 110 (26.6) | 831 (11.2) |
| Atrial fibrillation | 466 (15.2) | 90 (21.8) | 1186 (16.0) | |
| Chronic renal failure | 437 (14.3) | 4316 (7.6) | 102 (24.7) | 912 (12.3) |
| Cancer | 529 (17.3) | 7770 (13.7) | 104 (25.2) | 1378 (18.6) |
| Asthma | 285 (9.3) | 3388 (6.0) | 26 (6.3) | 387 (5.2) |
| Current smoker | 809 (26.4) | 8532 (15.0) | 88 (21.3) | 890 (12.0) |
| Antiplatelets | ||||
| Triflusal* | 24 (0.8) | 215 (0.4) | 6 (1.5) | 46 (0.6) |
| Aspirin* | 515 (16.8) | 8396 (14.8) | 113 (27.4) | 1496 (20.2) |
| Clopidogrel* | 83 (2.7) | 1162 (2) | 22 (5.3) | 252 (3.4) |
COPD, chronic obstructive pulmonary disease.
Data are presented as No. (%) or median [interquartile range].
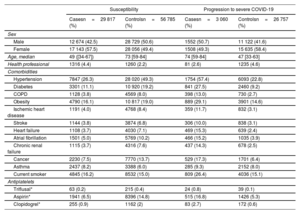
Demographic and clinical cohort characteristics (susceptibility and progression to severe COVID-19)
| Susceptibility | Progression to severe COVID-19 | |||
|---|---|---|---|---|
| Casesn=29 817 (%) | Controlsn=56 785 (%) | Casesn=3 060 (%) | Controlsn=26 757 (%) | |
| Sex | ||||
| Male | 12 674 (42.5) | 28 729 (50.6) | 1552 (50.7) | 11 122 (41.6) |
| Female | 17 143 (57.5) | 28 056 (49.4) | 1508 (49.3) | 15 635 (58.4) |
| Age, median | 49 ([34-67]) | 73 [59-84] | 74 [59-84] | 47 [33-63] |
| Health professional | 1316 (4.4) | 1260 (2.2) | 81 (2.6) | 1235 (4.6) |
| Comorbidities | ||||
| Hypertension | 7847 (26.3) | 28 020 (49.3) | 1754 (57.4) | 6093 (22.8) |
| Diabetes | 3301 (11.1) | 10 920 (19.2) | 841 (27.5) | 2460 (9.2) |
| COPD | 1128 (3.8) | 4569 (8.0) | 398 (13.0) | 730 (2.7) |
| Obesity | 4790 (16.1) | 10 817 (19.0) | 889 (29.1) | 3901 (14.6) |
| Ischemic heart disease | 1191 (4.0) | 4768 (8.4) | 359 (11.7) | 832 (3.1) |
| Stroke | 1144 (3.8) | 3874 (6.8) | 306 (10.0) | 838 (3.1) |
| Heart failure | 1108 (3.7) | 4030 (7.1) | 469 (15.3) | 639 (2.4) |
| Atrial fibrillation | 1501 (5.0) | 5769 (10.2) | 466 (15.2) | 1035 (3.9) |
| Chronic renal failure | 1115 (3.7) | 4316 (7.6) | 437 (14.3) | 678 (2.5) |
| Cancer | 2230 (7.5) | 7770 (13.7) | 529 (17.3) | 1701 (6.4) |
| Asthma | 2437 (8.2) | 3388 (6.0) | 285 (9.3) | 2152 (8.0) |
| Current smoker | 4845 (16.2) | 8532 (15.0) | 809 (26.4) | 4036 (15.1) |
| Antiplatelets | ||||
| Triflusal* | 63 (0.2) | 215 (0.4) | 24 (0.8) | 39 (0.1) |
| Aspirin* | 1941 (6.5) | 8396 (14.8) | 515 (16.8) | 1426 (5.3) |
| Clopidogrel* | 255 (0.9) | 1162 (2) | 83 (2.7) | 172 (0.6) |
COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 19.
Data are presented as number (%) or median [interquartile range].
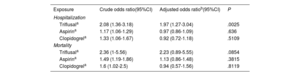
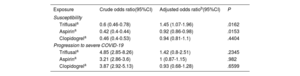
For the purposes of the analysis, the study included 3 060 cases with a positive PCR test who had been hospitalized. The control group consisted of 56 785 controls without a positive PCR test. After adjustment, triflusal (OR, 1.97; 95%CI, 1.27-3.04) appeared to increase the risk of hospitalization. In contrast, the use of aspirin (OR, 0.97; 95%CI, 0.86-1.09) and clopidogrel (OR, 0.92; 95%CI, 0.72-1.18) showed no statistical risk effect (table 3). The main results can be found in figure 1.
Risk of COVID-19 severity, requiring hospitalization, mortality and current antiplatelet use
| Exposure | Crude odds ratio(95%CI) | Adjusted odds ratiob(95%CI) | P |
|---|---|---|---|
| Hospitalization | |||
| Triflusala | 2.08 (1.36-3.18) | 1.97 (1.27-3.04) | .0025 |
| Aspirina | 1.17 (1.06-1.29) | 0.97 (0.86-1.09) | .636 |
| Clopidogrela | 1.33 (1.06-1.67) | 0.92 (0.72-1.18) | .5109 |
| Mortality | |||
| Triflusala | 2.36 (1-5.56) | 2.23 (0.89-5.55) | .0854 |
| Aspirina | 1.49 (1.19-1.86) | 1.13 (0.86-1.48) | .3815 |
| Clopidogrela | 1.6 (1.02-2.5) | 0.94 (0.57-1.56) | .8119 |
95%CI, 95% confidence interval.
Use of one type of anticoagulant does not exclude the fact that the patient may use another type of anticoagulant.
Adjusted for age, sex, and comorbidities: hypertension, diabetes, COPD (chronic obstructive pulmonary disease), obesity, ischaemic heart disease, stroke, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker and current use of other pharmacological treatments (antihypertensives, diuretics, nonsteroidal anti-inflammatory drugs, hypolipidemic agents, anticoagulants, and glucocorticoids). Polypharmacy (number of different medications prescribed and dispensed for chronic diseases in the last 6 months before the index date) was used as a proxy for the degree of chronicity in patients.
For the purposes of this analysis, there were 413 hospitalized cases with a positive PCR who died, and 7408 controls without a positive PCR test. No significant result was found for any of the antiplatelets, ie, triflusal (OR, 2.23; 95%CI, 0.89-5.55), aspirin (OR, 1.13; 95%CI, 0.86-1.48), and clopidogrel (OR, 0.94; 95%CI, 0.57-1.56) (table 3).
SusceptibilityFor the purposes of this analysis, there were 29 817 cases with a positive PCR (3060 hospitalized and 26 757 not hospitalized) and 56 785 controls without a positive PCR test. After adjustment, triflusal apparently increased the risk of virus infection (OR, 1.45; 95%CI, 1.07-1.96), whereas aspirin (OR, 0.92; 95%CI, 0.86-0.98) seemed to decrease susceptibility to COVID-19 infection. Use of clopidogrel (OR, 0.94; 95%CI, 0.81-1.1) showed no statistically significant effects on risk (table 4).
COVID-19 susceptibility, risk of progression to severe COVID-19, and current antiplatelet use
| Exposure | Crude odds ratio(95%CI) | Adjusted odds ratiob(95%CI) | P |
|---|---|---|---|
| Susceptibility | |||
| Triflusala | 0.6 (0.46-0.78) | 1.45 (1.07-1.96) | .0162 |
| Aspirina | 0.42 (0.4-0.44) | 0.92 (0.86-0.98) | .0153 |
| Clopidogrela | 0.46 (0.4-0.53) | 0.94 (0.81-1.1) | .4404 |
| Progression to severe COVID-19 | |||
| Triflusala | 4.85 (2.85-8.26) | 1.42 (0.8-2.51) | .2345 |
| Aspirina | 3.21 (2.86-3.6) | 1 (0.87-1.15) | .982 |
| Clopidogrela | 3.87 (2.92-5.13) | 0.93 (0.68-1.28) | .6599 |
95%CI, 95% confidence interval; COVID-19, coronavirus disease 19.
Use of one type of anticoagulant does not exclude the possibility that the same patient may use another type of anticoagulant.
Adjusted for: age, sex, and comorbidities: hypertension, diabetes, COPD (chronic obstructive pulmonary disease), obesity, ischemic heart disease, stroke, heart failure, atrial fibrillation, chronic renal failure, cancer, asthma, current smoker and current use of other pharmacological treatments (antihypertensives, diuretics, nonsteroidal anti-inflammatory drugs, hypolipidemic agents, anticoagulants, and glucocorticoids). Polypharmacy (number of different medications prescribed and dispensed for chronic diseases in the last 6 months before the index date) was used as a proxy for the degree of chronicity in patients.
For this analysis, there were 3060 cases with a positive PCR test who were hospitalized and 26 757 controls with PCR who were not hospitalized. After adjustment, use of triflusal (OR, 1.42; 95%CI, 0.8-2.51), aspirin (OR, 1; 95%CI, 0.87-1.15) and clopidogrel (OR, 0.93; 95%CI, 0.68-1.28) showed no statistically significant effects on risk (table 4).
DISCUSSIONThe results of this large-scale case-control study, based on real-world data, seem to indicate that, while chronic use of low-dose aspirin (LDA) or clopidogrel does not increase risk of hospitalization or COVID-19 mortality, it appears to be associated with a reduction in susceptibility to the virus. Conversely, triflusal could be associated with an almost 2-fold increased risk of hospitalization and mortality. In the case of mortality, this increase was not statistically significant. Hence, our findings suggest that, in COVID-19 pandemic situations, consideration should be given to avoiding prescription of triflusal and replacing it with clopidogrel and/or aspirin.
In the literature, some studies have analyzed the association between chronic antiplatelet use and susceptibility to the virus,28,29 while others have examined its association with disease severity, on the one hand, or risk of COVID-19 mortality,9,17,18 on the other. To our knowledge, however, ours is the first study to address all these outcomes at the same time.
Although aspirin and triflusal are salycilates that inhibit cyclooxygenase 1 (COX-1) and cyclooxygenase 2 (COX-2), triflusal inhibits COX-2 more mildly. In addition, triflusal also inhibits cAMP and cGMP.30 This could explain why they play a different role in COVID-19 severity. Triflusal is not approved by the European Medicines Agency or the United States Food and Drug Administration but is currently marketed in 25 countries.31 The detection of possible risks associated with its chronic use could therefore be highly relevant from both a clinical and public health perspective. While it might be conjectured that this difference in effect could be because the recommended doses of triflusal as an antiplatelet are higher than those of aspirin,32,33 we believe that this difference would not account for an effect magnitude as high as that found for risk of hospitalization. Similarly, this would not be accounted for by the presence of confounding by indication, since both drugs share therapeutic indications.32,33 Along with their similar chemical structure, it is however possible that our findings may be explained by an independent pleiotropic effect of the mechanism of action specific to both antiplatelets.
Our results seem to indicate that chronic use of LDA or clopidogrel has no significant effects on risk of hospitalization, COVID-19 mortality, or progression to more severe disease stages, in line with some already published studies.9,17 Our study data also reveal that chronic use of LDA is apparently associated with a reduction in susceptibility to the virus (OR, 0.92; 95%CI, 0.86-0.98), a finding in line with those of other earlier studies.12,29 With respect to mortality, the results of previous studies on LDA use at the prehospital level are inconsistent with regard to its ability to reduce COVID-19 mortality: whereas some studies point to a protective effect, others fail to find significant effects.3,15 It is, however, true that chronic use (even prior to virus infection) is not the same as prehospital use in which LDA is administered once the patient has been diagnosed with COVID-19 in the early stages.
In our study, chronic triflusal use was apparently associated with a statistically significant increased risk of hospitalization (OR, 1.97; 95%CI, 1.27-3.04) and a nonstatistically significant increased risk of mortality (OR, 2.23; 95%CI, 0.89-5.55). A similar effect for mortality was described by Soldevila et al.,34 who found that the risk among triflusal users was twice as high as that of aspirin users (60.0% vs 28.4%). We were unable to find any more studies on the effects of triflusal, possibly because its use is less widespread as that of aspirin.35 However, its use is by no means inconsiderable, since it is approved in many countries.31 The high effect magnitude found between triflusal and risk of hospitalization, taken together with the nonsignificant association with mortality, would indicate the need to seek alternative treatments in pandemic situations.
Advantages and limitationsOne of the most important strengths of our study is its large sample size (more than 86 000 participants), achieved by exhaustive sampling, which enabled us to include all cases with a diagnosis of COVID-19 in Galicia in 2020.
Another strong point is the adjustment made for the presence of confounding variables (sociodemographic data, comorbidities, and use of other drugs). This allowed us to estimate the possible association between chronic use of each type of antiplatelet and the COVID-19 disease process with precision.
Furthermore, our measurement of the variable of exposure was based on data corresponing to drugs actually dispensed at pharmacies, and are not based on prescriptions (as in other studies), thus reducing the risk of misclassification.
Nevertheless, the existence of residual confounding due to unmeasured or misclassified study variables cannot be completely ruled out, given that we had no data that could measure the severity of comorbidities associated with greater COVID-19 severity. However, this occurs in all observational studies in which secondary databases may be available.
Furthermore, controls were not matched with cases in the cohorts used to study susceptibility and progression to severe COVID-19. Nonetheless, many authors report that this in no way diminishes the validity of studies, since the absence of matching might impair the efficiency of a study but does not increase the risk of bias.20,23 Moreover, in 2020, hospitalizations would have been proportional to COVID-19 cases.
The lack of sufficient diagnostic tests in the initial months of the pandemic could have led to some of the asymptomatic COVID-19 participants being assigned to controls. Some participants who did not have a PCR test may have actually had COVID-19 and therefore there could be a misclassification in the output. However, we have no reason to believe that this misclassification would differ between the exposure groups (triflusal, aspirin and clopidogrel, no antiplatelet), and therefore the effect estimates would not be biased.36 In addition, the viral load was not detected in these tests. Due to the low number of participants taking triflusal, no conclusive results were obtained in the dose-response analysis. The database we used in our study lacked information on the indications of the prescribed treatments, and therefore we could not adjust for potential indications. Finally, race was not included as a covariate. However, in our geographical area, practically the entire population is Caucasian.
CONCLUSIONSOur results suggest that neither clopidogrel nor LDA are associated with an increased risk of hospitalization or mortality, and that LDA even seems to be associated with a decrease in susceptibility. In contrast, triflusal seems to be associated with an appreciable rise in the risk of hospitalization and mortality. These results indicate that, while suspending antiplatelet treatment with clopidogrel or LDA is not be warranted, triflusal prescription in COVID-19 pandemic situations should be reconsidered. If these results are reproduced with other databases and replicated in clinical trials, we believe that they could be relevant in the prescription of antiplatelet agents in pandemic situations.
FUNDINGThis work was supported by the Carlos III Institute of Health via the [COV20/00470] project (cofunded by the European Regional Development Fund, “A way to make Europe”).
ETHICAL CONSIDERATIONSThis study was approved by the Galician Clinical Research Ethics Comittee (Comité de Ética de Investigación de Galicia, reference 2020/349), certified by the Spanish Agency for Medicines and Medical Devices (AEMPS), and conducted in accordance with the principles of the Declaration of Helsinki and current legislation on biomedical research. The study protocol is registered in the EU Electronic Register of Post-Authorisation Studies and is available online at https://www.encepp.eu/encepp/viewResource.htm?id=44588. Automated data extraction was performed and anonymized to ensure that the participants could not be identified and therefore it was not necessary to obtain informed consent from the participants.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence was used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSA. Prieto-Campo designed study and wrote the paper. M. Zapata-Cachafeiro designed the study and wrote the paper. M. Portela-Romero designed study and revised the paper. M. Piñeiro-Lamas designed study, analyzed data and revised the paper. A. Figueiras designed the study, analyzed data, and revised the paper. A. Salgado-Barreira designed the study, analyzed data and revised the paper. All authors read and approved the final version.
CONFLICTS OF INTERESTNone.
- -
SARS-CoV-2 gives rise to endothelial dysfunction and platelet and neutrophil activation, which induces a state of hypercoagulation and inflammation.
- -
SARS-CoV-2 increases the risk of venous and arterial thromboebolism, which could in turn lead to multiorgan damage, increasing the risk of mortality.
- -
LDA and clopidogrel did not appear to increase the risk of hospitalization or COVID-19 mortality. Conversely, these drugs were associated with a reduction in susceptibility to the virus.
- -
Triflusal was associated with an almost 2-fold increase in the risk of hospitalization and mortality; in the case of mortality, this increase was not statistically significant.
We would like to thank the SERGAS General Healthcare Directorate for providing the data needed to conduct this study, DXC Technology for its work in extracting the study data, and Michael Benedict for reviewing and revising the English.