The guidelines for the management of atrial fibrillation (AF) incorporate new risk factors for thromboembolism, trying to de-emphasize the use of the ‘low’, ‘moderate’, and ‘high’ risk categories. The objective of this study was to determine the impact of the new scheme CHA2DS2-VASc and of the new recommendations for oral anticoagulation (OAC) in a contemporary sample of patients with AF seen by primary physicians and cardiologists.
MethodsMulticenter, observational, cross-sectional study on the epidemiology of hypertension and its control, designed by the arterial hypertension department. Each researcher enrolled the first 6 consenting patients who came for examination during a 5-day period.
ResultsOf 25 137 individuals recruited, 1544 were diagnosed with AF. The vast majority of the sample had a CHADS2 score ≥2 (77.3%). Individuals with a risk score lower than 2 were categorized according to the CHA2DS2-VASc score: 14.4% were aged 75 years or older (CHA2DS2-VASc=2). Of those younger than 75, 42.3% had a CHA2DS2-VASc=2; 23.7% CHA2DS2-VASc=3, and 1.1% CHA2DS2-VASc=4. This means that the 85.1% of the patients with a CHADS2 score<2 and no contraindications are indicated for OAC.
ConclusionsThe new recommendations will result in a significant increase in patients with indications for OAC, at the expense of those previously characterized as low-to-moderate risk. Therefore, patients at risk of thromboembolic events must be identified, although an evaluation of bleeding risk should be part of the patient assessment before starting anticoagulation.
Keywords
.
INTRODUCTIONOne of the greatest challenges in the management of patients with nonvalvular atrial fibrillation (AF) is the identification of risk factors for developing thromboembolic complications. This has led to the publication within the past decade of several algorithms for risk stratification that categorize patients based on risk of embolism.1, 2, 3, 4, 5 The most widely used system is the CHADS2 scale, an acronym for these established risk factors3: congestive heart failure (HF), arterial hypertension (AHT), age ≥75 years, diabetes mellitus (DM), and ischemic stroke (IS). Each factor is worth 1 point, except for a history of IS, which is worth 2. However, this scale has certain limitations, especially in low-to-moderate risk patients, many of whom would benefit from oral anticoagulation (OAC) treatment. Furthermore, this scale does not consider other risk factors.6
The guidelines set forth by the European Society of Cardiology in 20107 emphasize other “modulating” factors that were previously not taken into consideration, such as vascular disease or female sex, and it proposes a new classification system for age, which is divided into 3 categories (<65 years, 65 to 74 years, and ≥75). The new “CHA2DS2-VASc” scale reclassifies a substantial number of patients previously considered “low” or “moderate” risk into higher categories. This would increase the indication for OAC, although the true magnitude of these recommendations has not yet been quantified. The aim of our study was to analyze the impact of classifying patients with AF according to the CHA2DS2-VASc scale in comparison to the CHADS2 scale, and to test the new OAC recommendations in a sample of patients with AF recruited from primary care and cardiology outpatient clinics. The outcomes were also compared with the results from the 1999 CARDIOTENS patient registry, which had a similar design.8
METHODS Study DesignWe performed a multicenter, cross-sectional, observational, epidemiological study designed by the Spanish Society of Cardiology Working Group on Arterial Hypertension. The aim of the study, supported by the Research Agency of the SEC, was to assess the prevalence and level of control of AHT in common clinical practice. Patients were recruited from outpatient cardiology and primary care clinics. Inclusion criteria were age ≥18, access to all past clinical histories and diagnoses of cardiovascular pathologies; and signed informed consent from the patient or the patient's legal representative to participate in the study. The exclusion criteria were addiction to or consumption of illegal drugs (cocaine, cannabis, psychotropic drugs) and refusal to participate. We selected 885 physicians, 89.1% from primary care and 10.9% from cardiology. Each participating physician included in the study the first 6 patients complying with inclusion criteria during each workday for a period of 5 days. In total, 25 137 patients were recruited: 15 102 (60.1%) had some type of cardiovascular disease or risk factor and 1544 (6.14% of the total, 95% confidence interval [CI]: 6.13-6.15) were diagnosed with AF. We developed a 3-page study questionnaire that was given to each patient. As in the 1999 CARDIOTENS registry,8 the first page requested information on sociodemographics, cardiovascular disease, cardiovascular risk factors, and patient history. In the case of positive answers to any of the sections regarding diagnosed cardiovascular disease (ischemic heart disease, angina or HF, or IS) or the presence of risk factors (dyslipidemia, AHT, DM, or tobacco use), a second page was also filled out. The final page included more detailed information regarding treatment, electrocardiogram and echocardiogram results, and laboratory analyses. Only electrocardiogram and/or laboratory results for the previous 6 months were eligible. Blood pressure and heart rate were measured during the visit.
VariablesThe CHADS2 scale assigns 1 point for the presence of each of the following factors: HF, AHT, age >65 years, and DM, and 2 points are given for a history of IS; thus the maximum score is 6. The CHA2DS2-VASc awards 1 point for the presence of HF (or left ventricular dysfunction), AHT, DM, peripheral vascular disease (including myocardial infarction, complex aortic plaques, and peripheral arterial disease), age between 65 and 74 years, and female sex, and 2 points for age ≥75 years and previous IS.7
We defined AHT as cases in which 2 consecutive blood pressure measurements recorded ≥140/90mmHg or the patient was taking specific antihypertensive treatment. Controlled AHT was defined as complying with the objectives set forth in the 2009 re-evaluation guidelines (<140/90mmHg). A background of DM was deemed a previous diagnosis of DM registered in the patient's clinical history, specific drug treatment for DM, or 2 consecutive measurements of fasting blood glucose >126mg/dl. We considered that the patient had a background of AF if a medical report or electrocardiogram showed it. Ischemic heart disease was defined as cases with a previous history of acute myocardial infarction, stable or unstable angina, percutaneous or surgical coronary revascularization, or positive ischemia induction (stress test, scintigraphy, stress echocardiography, etc.). The HF score was assigned to patients with at least 1 previous hospitalization for HF as recorded in the patient discharge report, as well as those patients with signs and symptoms of HF and compatible imaging test results (chest X-ray or echocardiogram). A background of IS was defined as cases in which some type of ischemic, hemorrhagic, or temporary stroke was recorded in the patient history or any medical record. A background of intermittent claudication, revascularization of the legs, amputation, or established diagnosis was defined as peripheral arterial disease. Ischemic heart disease, HF, peripheral arterial disease, and IS were included in the CVD definition.
Statistical AnalysisWe used SPSS statistical software version 15.0 for all data analyses (SPSS Inc., Chicago, Illinois). All variables maintained a normal distribution, and have been summarized as mean (standard deviation). Proportions were compared using Student's t-tests and chi-square tests in order to evaluate statistical differences between the medical treatment provided to patients with and without AF. The use of OAC for the CARDIOTENS 1999 and the CARDIOTENS 2009 registries was compared using the Student's t and the estimated percentages for each registry to calculate variance. We set the level of statistical significance at P≤.05.
RESULTSOf the 25 137 patients that participated in the study, 1544 (6.14% of the total and 10.22% of patients with risk factors or CVD) had a history of AF (persistent, permanent, or paroxysmal). Table 1 shows that patients with AF had higher mean age and prevalence of risk factors and CVD. Patients with AF also received more OACs, antiplatelets, betablockers, antihypertensives, nitrates, calcium antagonists, diuretics, and statins (Table 2).
Table 1. Baseline Characteristics of Patients With and Without Atrial Fibrillation.
| Total | Without AF | With AF | P | |
| Total (n) | 25 137 | 23 593 (93.5%) | 1544 (6.5%) | |
| Age (years) a | 61 (15.51) | 52 (15.4) | 73 (10.8) | <.01 |
| Heart rate (beats/min) | 73 (10.84) | 73 (10.42) | 74 (13.45) | .11 |
| SBP (mmHg) | 135.6 (15.48) | 135.51 (15.2) | 135.85 (16.59) | .44 |
| DBP (mmHg) | 78.7 (11) | 78.74 (11.31) | 78.33 (12.86) | .19 |
| BMI a | 28.35 (4.82) | 28.29 (4.82) | 28.81 (4.91) | <.01 |
| Abdominal perimeter (cm) a | 96.42 (14.27) | 96.24 (14.13) | 97.26 (14.62) | <.01 |
| Glucose (mg/dl) a | 110.9 (36.67) | 110.16 (35.8) | 115.77 (40.71) | <.01 |
| Total cholesterol (mg/dl) a | 204 (44.62) | 204.82 (44.62) | 197.69 (43.48) | <.01 |
| LDLc (mg/dl) a | 123.33 (37.25) | 123.8 (37.32) | 119.7 (35.89) | <.01 |
| HDLc (mg/dl) a | 52.73 (15.83) | 53.08 (15.79) | 50.32 (15.5) | <.01 |
| Triglycerides (mg/dl) | 142.13 (69.97) | 141.67 (69.6) | 144.06 (72.4) | .23 |
| Uric acid (mg/dl) a | 5.44 (1.58) | 5.38 (1.55) | 5.91 (1.68) | <.01 |
| Creatinine (mg/dl) a | 0.72 (0.78) | 0.7 (0.74) | 0.83 (0.71) | <.01 |
| Glycosylated haemoglobin (%) | 6.25 (1.43) | 6.28 (1.41) | 6.31 (1.44) | .3 |
AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; HDLc, high-density lipoprotein cholesterol; LDLc, low-density lipoprotein cholesterol;SBP, systolic blood pressure
The first column represents the number of patients in which the given variable was registered. Values are expressed as mean (standard deviation)
a Indicates variables in which differences were statistically significant.
Table 2. Patient Treatment Based on the Presence or Absence of Atrial Fibrillation.
| Total | Without AF | With AF | P | |
| Patients | 25 137 | 23 596 | 1 544 | |
| OAC | 1372 (5.5) | 404 (1.7) | 968 (62.7) | <.01 |
| Acetylsalicylic acid | 4055 (16.1) | 3579 (15.2) | 476 (30.8) | <.01 |
| Clopidogrel | 1175 (4.7) | 995 (4.2) | 180 (11.7) | <.01 |
| Betablockers | 2937 (11.7) | 2297 (9.7) | 640 (41.5) | <.01 |
| ACE-inhibitor | 4736 (18.8) | 4108 (17.4) | 628 (40.7) | <.01 |
| ARB II | 3615 (14.4) | 3076 (13) | 539 (34.9) | <.01 |
| Diuretics | 509 (2) | 424 (1.8) | 85 (5.5) | <.01 |
| CA | 3615 (14.4) | 3076 (13) | 539 (34.9) | <.01 |
| Nitrates | 1281 (5.1) | 1014 (4.3) | 267 (17.3) | <.01 |
| Statins | 6509 (25.9) | 5695 (24.1) | 814 (52.7) | <.01 |
CA, calcium antagonist; OAC, oral anticoagulant; ARB II, Angiotensin II receptor blockers; AF, atrial fibrillation; ACE, angiotensin converting enzyme
Data are expressed as no. (%)
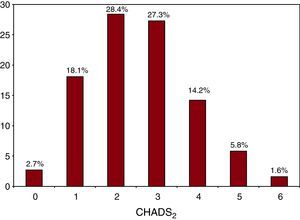
Of all 1544 patients with AF, 1193 (77.3%) had a moderate to high risk profile (CHADS2 score ≥2), with scores of 2 and 3 being the most frequent (28.4% and 27.3%, respectively); 299 patients had a score of 1 (18.1%) and only 41 patients (2.7%) had low risk (CHADS2=0) (Figure 1). Of the patients with a CHADS2 score ≥2, 35.5% were not receiving OAC treatment (n=148) and 70.9% of this subgroup were older than 65 years, 56% were older than 74 years, and 53.5% were women. In this subgroup of high-risk patients receiving no OAC treatment, the preferred alternative therapy was antiplatelet medication with acetylsalicylic acid or clopidogrel (67.4%), or both (8.9%); 23.7% received no drug treatment. Lastly, patients with CHADS2 ≥2 being treated in primary health care settings were less likely to receive anticoagulation treatment than those treated by cardiologists (64.5% vs 35.5%, P=.08).
Figure 1. Percentage of patients in each group according to the CHADS2 scale.
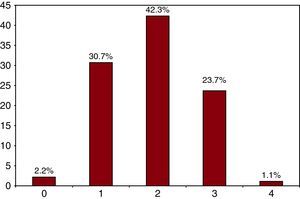
According to the CHADS2 scale, 41.7% of the low-to-moderate risk population (score 0 or 1) was not receiving OAC, and most of the patients in this subgroup were aged 75 and older (63.4%, vs 36.6% of the entire ≥75 age group and 56.8% vs 43.2% in the group aged <75 years). Just 14.4% of the low-to-moderate risk patients were aged 75 and older (thus CHA2DS2-VASc score=2), and 40% were between 65 and 74 years. In the subgroup of patients younger than 75 years with CHADS2 <2, 67.1% had 2 or more risk factors (42.3% CHA2DS2-VASc score=2, 23.7% CHA2DS2-VASc score=3, and 1.1% CHA2DS2-VASc score=4); 30.7% had 1 risk factor (CHA2DS2-VASc score=1), and only 2.2% had no risk factors (CHA2DS2-VASc score=0) (Figure 2).
Figure 2. Percentage of risk factors in patients younger than 75 years with a CHADS2 score l<2.
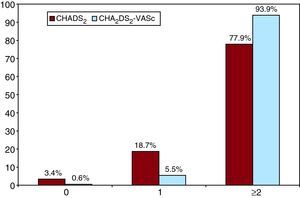
As such, using the new risk scale, with the redefined category of low or moderate risk, the percentage of patients with indication for anticoagulation treatment (in the absence of contraindications) would be 81.5% (67.1% of those younger than 75 years and 14.4% of those with age ≥75 as their only risk factor). If the high-risk population were added (77.3%), then 93.8% of patients with AF would have indications for OAC (Figure 3). As a consequence, 1.8% of patients would be able to choose between this type of therapy and antiplatelet treatment.
Figure 3. Final result after categorising patients according to the CHA2DS2-VASc in comparison with the CHADS2 scale.
DISCUSSIONThe results from this analysis of the CARDIOTENS 2009 study show the high prevalence of AF in patients attended by specialists, and the consequent increase in the number of patients eligible for OAC treatment according to the new recommendations and stratification system for risk of embolism. The mean age, prevalence of risk factors, and prevalence of AF are similar to the values from previous national and international registries, and so our results may be representative of normal clinical activity. In addition, this is the first time that an analysis has been carried out on the impact of new criteria for anticoagulation treatment in patients with AF in a large contemporary cohort of patients diagnosed with AF in Spain.
Compared to the CARDIOTENS 1999 registry, we observed an increase in the number of patients being treated with OAC,9 from 33% (95% CI: 32-34) to 62.7% (95% CI: 59.9-65.5) in 2009, which is a 90% increase in one decade (P<.01). Another result that stands out is the large majority of the study population that had a moderate-to-high risk profile for thromboembolic events (77.3% had a CHADS2 ≥2). In spite of this, a high percentage of high-risk patients (35%) are not taking anticoagulants, which is a more severe problem in patients at highest risk, such as women and the elderly. However, this underuse of OAC is lower than the rates observed in other medical registries.10 Contraindications for OAC partially explain this situation, as well as the lack of medical indication and patient compliance due to the need for close monitoring and/or fear of bleeding. Meanwhile, within this subgroup of patients at risk for thromboembolism without OAC therapy, the preferred strategy is monotherapy with acetylsalicylic acid, even though this treatment is less effective than OAC in reducing thromboembolism, both in monotherapy11 and together with clopidogrel.12 Furthermore, this treatment also produces high rates of bleeding. Perhaps with new anticoagulant alternatives that are easier to manage, we will be able to mitigate these problems to some extent, thus increasing the use of OAC in patients for whom this therapy is indicated. We can also deduce from this registry that a high percentage of patients would have indications for OAC under the new recommendations, over 90% if we extrapolate the data from this sample to the general population.
This increase in the percentage of patients with indications for OAC is obviously at the expense of the patients previously categorized as low-to-moderate risk. This strategy is supposed to lead to a reduction in the risk of thromboembolic events, but also to an increase in the overall number of bleeding events. Thus it will be more crucial now than ever to identify this at-risk population, to minimize to the extent possible the potential risks inherent to the treatment, especially in the elderly.13 The new clinical guidelines can be of help on this point, as they provide bleeding risk scales that must be taken into account when it comes to evaluating a treatment strategy. Current recommendations continue to leave the choice of prescribing OAC or antiplatelet therapy to the physician within a certain range of patients, specifically those with a CHA2DS2-VASc score of 1 (although OAC is recommended). In any case, this will be a much lower percentage of patients (1.8% in this registry) because many patients will have clear indications for anticoagulants in the absence of contraindications.
We observed a paradox in that, although the great majority of medical registries have shown consistent underuse of anticoagulants,10 each new edition of the clinical guidelines for patients with AF widens the indications for this type of treatment. This has not gone without its critics by those who believe that the recommendations set forth are too exhaustive, with no clear benefits demonstrated in randomized clinical trials for each specific scenario,14 and that the guidelines have been established without conclusive data supporting improved patient prognosis as compared to the previous CHADS2 scale. Although some markers are easy to define, such as age or sex, vascular disease represents a much more diffuse and heterogeneous criterion that is difficult to apply to the general population.
Although it appears that these guidelines extend the indications for OAC, it is important to establish the need to minimize or avoid their association with antiplatelet drugs in patients with AF and ischemic heart failure (these two conditions coexist in 20% to 30% of patients),15 shortening the duration of triple therapy following a stent implantation: 1 month for non-drug-eluting stents and 3 to 6 months for drug-eluting stents or in the presence of ACS. In patients with a HAS-BLED bleeding risk score ≥3, non-drug-eluting stents are recommended, followed by treatment with acetylsalicylic acid, clopidogrel, and OAC for 2-4 weeks, or 4 weeks in the case of ACS.7 As such, we would expect to observe lower rates of bleeding in this particular subgroup than the present values.
LimitationsOur study has some limitations that must be taken into consideration. In the first place, this is a cross-sectional study, and we could not establish a timeline for each disease. This could be especially limiting in the case of AF, which can vary based on the treatment strategy employed (controlling rate or frequency), the evolution of the disease and treatment-related complications (embolism, bleeding, hospitalization, surgery, etc.). Moreover, the CARDIOTENS registry population design does not include a randomized and representative sample, but only those patients attended to by specialists, and so the conclusions of the study can only be considered in that context. Another limitation has been the inability to calculate the bleeding risk, as not all variables required to perform the HAS-BLED calculations were available.7 Lastly, thromboembolic risk could not be calculated in a small portion of patients due to loss of data, although this percentage was very small (1.9%) and we do not believe this had any effect on our conclusions.
CONCLUSIONSThe new indications for anticoagulant treatment will imply a significant increase in the number of patients with indications for this type of therapy, at the expense of those previously categorized as low-to-moderate risk. Therefore, patients at risk of thromboembolic events must be identified but without losing sight of the bleeding risk profile in order to compensate, to the extent possible, for the treatment side effects.
FUNDINGCARDIOTENS study has an unconditional grant from Laboratorios Recordati España.
CONFLICTS OF INTERESTNone declared.
Received 11 December 2010
Accepted 7 March 2011
Corresponding author: Heart Rhythm Management Centre, University Hospital Brussels-UZ Brussels, Laarbeeklaan 101, BE-1090 Brussels, Belgium. mrodrigu@uzbrussel.be