Left atrial appendage closure can be an attractive option for patients with nonvalvular atrial fibrillation and a contraindication to oral anticoagulants, provided that satisfactory results can be achieved during implantation and follow-up.
MethodsThirty-five consecutive patients, not eligible for randomized trials with oral anticoagulants, had an Amplatzer occlusion device implanted under general anesthesia. After the first 5 patients, 3-dimensional imaging was incorporated. The results of the implantation and the follow-up were analyzed over a 1-year period.
ResultsThe mean age was 74.65 (7.61) years, with a CHADS2 score of 2.41 (1.53) and a CHA2DS2-VASc score of 3.17 (1.60). Implantation failed in 1 patient and 5 needed a change in the selected plug size. There were no cardiac complications during the implantation or hospital stay. There was 1 vascular complication (arteriovenous fistula). Transesophageal echocardiography monitoring was performed at 24h, 1, 3, 6, and 12 months and we found 5 thrombi which were resolved with heparin. In the follow-up period of 21.14 (10.09) months, 3 patients aged>80 years died, none of them due to heart problems, and one transient ischemic stroke without further consequences.
ConclusionsLeft atrial appendage closure by an experienced operator can be a treatment option with few complications and with efficient results at>1 year in reducing thromboembolic and hemorrhagic complications, even in very high-risk groups.
Keywords
Nonvalvular atrial fibrillation (AF) is a frequent arrhythmia whose incidence increases with age, with rates greater than 10% in patients aged>80 years.1,2
Apart from hemodynamic repercussions, the importance of AF lies in the fact that it is responsible for more than 20% of ischemic ictus,3 AF being the cause of ischemic ictus with the greatest impact.1,3Until now oral anticoagulants (OAC) have been the main tool used to reduce cardioembolic incidents.4 However, due to the associated risk of hemorrhage they cannot be used in one third of patients.5,6 Although the development of new OACs (dabigatran, rivaroxaban, apixaban) has reduced some of the limitations of the original OACs (such as warfarin), especially by reducing intracranial hemorrhage and avoiding international normalized ratio monitoring, the percentage of major (2.15%-3.6% year) and minor hemorrhages (15%-20% year) has not changed significantly.7–9
The emergence of occlusion devices for the left appendage or left atrium appendage (LAA)10–12 presented a new treatment option for patients with contraindications to OACs, or with high risk of bleeding due to a high HAS-BLED (Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile INR, Elderly, Drugs/Alcohol Concomitantly) score,13 with similar results to OACs.11 It would be interesting to know the results of cohorts of patients who could not be included in the latest randomized studies of new OACs or PROTECT AF (Randomized Prospective Trial of Percutaneous LAA Closure vs Warfarin for Stroke Prevention in AF), since those patients have a higher overall risk of thromboembolic complications and hemorrhage both during the intervention and during the follow-up period.
The aim of our study was to present the results of our first 35 patients who had an occlusion device (Amplatzer Cardiac Plug [ACP], St Jude Medical Minnesota, United States) implanted, both during the intervention and during a 1-year follow-up period.
MethodsThirty-five patients recruited between March 2009 and November 2011 underwent LAA closure with an ACP device. The following conditions were required for inclusion: severe hemorrhage during treatment with acenocoumarol, a disease or previous clinical event that contraindicated the use of OAC, the repeated impossibility of monitoring the international normalized ratio, with instructions to suspend acenocoumarol by a hematologist. The study's protocol was accepted by the ethics committee for clinical research of our hospital and all patients provided informed consent. The aim of the study was to analyze the initial results of the safety of the technique for ACP implantation. We also analyzed the patients’ long-term clinical course, including the development of embolic or hemorrhagic events, the formation of thrombi in the device and their outcome, and the long-term effectiveness of LAA closure or reduction of any residual initial shunt.
Transesophageal echocardiography (TEE) was performed in each patient 24-48h before the intervention to rule out the presence of thrombus in the LAA. After the first 5 patients, we performed a magnetic resonance imaging (MRI) scan in 10 patients and a computed tomography (CT) scan in the last 20 patients some days prior to the intervention. One hour before, a broad spectrum antibiotic (cephalosporin) was administered. The procedure was carried out under general anesthetic. We administered 100 U/kg of heparin after transseptal puncture. Transseptal access to the left atrium was through the right femoral vein; then we performed selective angiography of the LAA with a volume similar to that of a left coronary artery and, in general, in right anterior oblique (RAO) (20-30) caudal (15-25) and also in cranial RAO (15-20) views. The standard method to correct amplification was a radiopaque ball in the plane of the midaxillary line. Measurements were taken with TEE during the intervention in 2 planes. The anatomy of the appendage anatomy was studied with a probe in the midesophageal position by means of a 0°-135° scan to obtain the minor (between 45°-70°) and major ostial diameters (approximately 135°). These measurements were usually performed in the ostium, above the corresponding point of the circumflex artery, and 5 mm-10mm deeper, taking into account variations in the shape and direction of the axis of some appendages.
Intraoperative TEE also allowed for the position of the device release sheath after transseptal puncture to be evaluated, the correct position of the device in the appendage to be confirmed, and the correct exclusion of the appendage before its release to be checked using the Doppler-colour display. A 3-dimensional (3D) echocardiogram was used in the final 14 patients to confirm the sealing of the margins of the ostium, to ensure that the device could not have affected adjacent anatomical structures, such as the implantation of the mitral valve or the upper left pulmonary vein, and to confirm the absence complications such as a pericardial effusion.
We performed MRI studies with a Signa 1.5-T model General Electric scanner. The slices were obtained without respiratory motion through a sequence of magnetic resonance angiography with fast gradients (to achieve the best spatial resolution) and in different planes (sagittal, coronal and transversal). Cardiac CT imaging was carried out using a 64-slice scanner (General Electric Light Speed VCT Healthcare Inc.; Milwaukee, WI, United States).
To improve the images taken by the radiology department, and in the absence of specific software, we considered the possibility of exporting these images without processing them through a CARTO software system (CartoMergeTM Image Integration Module, Biosense Webster, Inc.). By means of a 3D reconstruction process of the heart and segmentation, a 3D image of the left atrium and the LAA and the pulmonary veins was achieved, allowing for detailed internal and external analysis and for very precise measurement of distances in any direction in the area. This method has been validated in previously published studies.14,15
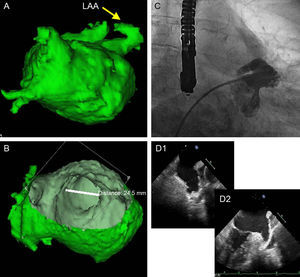
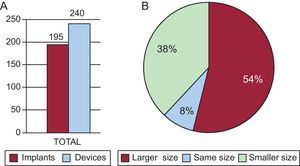
The TEE and angiography measurements were compared with the measurements obtained by CARTO-CT/MRI, with special attention to the major axis, which was usually the superoinferior (SI) axis. If the measurements differed in the size of the ACP which would correspond to the range (according to the table of equivalent measurements/size for the device provided by the company), then both the TEE and the angiography were performed again, paying special attention to the ostium and the implantation-anchorage area (landing zone), approximately 1cm within the ostium and from the inferior angle, precisely at the upper level of the circumflex artery. If the measurements did not match after a new evaluation, the consistency of the measurements and the quality of the images were checked, giving most weight to the 3D measurement of the CARTO-CT/MRI on the major axis (Fig. 1).
Work plan in our study. A: CARTO/computed tomography image of the left atrium and the left atrial appendage. B: Internal slice for measurements of the ostium of the left atrial appendage. C: Angiography image resembling the shape of the appendage seen in image A. D: Perpendicular slices using 2-dimensional transesophageal echocardiography of the left atrial appendage. LAA, left atrial appendage.
A follow-up TEE was performed on the following day and at 1, 3, 6, and 12 months in order to check the correct position of the device, the presence or absence of shunt in the interatrial wall and the absence of flow toward the LAA. We paid special attention to the possible presence of thrombus in the device. Adverse events of possible embolic or hemorrhagic origin and admissions due to concurrent illnesses were also considered.
A loading dose (600mg) of clopidogrel was administered as anticoagulant/antiplatelet treatment during device implantation, and 300mg of acetylsalicylic acid was administered on the first day followed by 100mg daily starting the day after the intervention. Clopidogrel was continued for 3 months, except when there were hemorrhagic complications, and acetylsalicylic acid was continued for 6 months. If a thrombus occurred, a therapeutic dose of subcutaneous enoxaparin was added for 2 weeks, clopidogrel was prolonged and the TEE was repeated to check for disappearance. When there was a negative result, the decision to prolong the treatment for another week or to hospitalize the patient and begin treatment with intravenous heparin was evaluated.
All procedures were carried out by the same surgeon and there were no problems with transseptal puncture, which consequently had no influence on other variables.
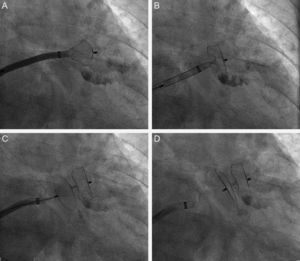
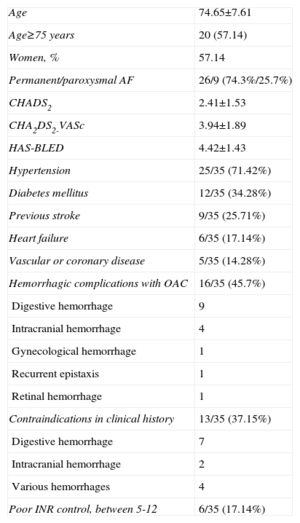
Successful implantation was defined as: a) positioning of the device with a satisfactory distance between the body and the exterior disc, i.e., the body was in the neck of the appendage and the disc covered the ostium, with a clear separation between both in the 2 usual angiographic views (cranial RAO and caudal RAO) or in the short axis of the TEE; b) an image of slight compression of the body (pneumatic type) but without the ‘raspberry’ effect indicative of excessive compression; c) absence of flow between the left atrium and the peri-device LAA or <3mm visible with the color Doppler scan; and d) test for firm anchorage of the device to the LAA by a manual ‘push and pull’ procedure, similar to the well-known ‘Minnesota wiggle’ (Fig. 2).
Phases of implantation and release of the Amplatzer Cardiac Plug device in the left appendage. A: Partial exit of the body or ‘lobe’ of the Amplatzer Cardiac Plug device toward the interior of the left appendage. B: Complete exit of the ‘lobe’. C: Complete exit of the lobe and the disc of the Amplatzer Cardiac Plug. D: Amplatzer Cardiac Plug device blocking the appendage after complete release from the sheath.
Table 1 shows the main characteristics of the 35 patients. None of these 35 patients could have entered randomized studies with OACs as these agentswere contraindicated for the various reasons outlined in Table 1.
Main Characteristics of the Patients (n=35).
| Age | 74.65±7.61 |
| Age≥75 years | 20 (57.14) |
| Women, % | 57.14 |
| Permanent/paroxysmal AF | 26/9 (74.3%/25.7%) |
| CHADS2 | 2.41±1.53 |
| CHA2DS2-VASc | 3.94±1.89 |
| HAS-BLED | 4.42±1.43 |
| Hypertension | 25/35 (71.42%) |
| Diabetes mellitus | 12/35 (34.28%) |
| Previous stroke | 9/35 (25.71%) |
| Heart failure | 6/35 (17.14%) |
| Vascular or coronary disease | 5/35 (14.28%) |
| Hemorrhagic complications with OAC | 16/35 (45.7%) |
| Digestive hemorrhage | 9 |
| Intracranial hemorrhage | 4 |
| Gynecological hemorrhage | 1 |
| Recurrent epistaxis | 1 |
| Retinal hemorrhage | 1 |
| Contraindications in clinical history | 13/35 (37.15%) |
| Digestive hemorrhage | 7 |
| Intracranial hemorrhage | 2 |
| Various hemorrhages | 4 |
| Poor INR control, between 5-12 | 6/35 (17.14%) |
AF, atrial fibrillation; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ration, elderly, drugs/alcohol concomitantly; INR, international normalized ratio; OAC, oral anticoagulants.
Data are expressed as no. (%) or mean±standard deviation.
The following CHADS2 scores were calculated: 2.41 (1.53), CHA2DS2-VASc: 3.17 (1.60) and HAS-BLED13: 4.42 (1.43).
Outcomes of the InterventionTable 2 shows the operating times, contrast volumes and time of radioscopy use, together with the measurements obtained by intraoperative TEE, angiography and CARTO-MRI or CARTO-CT. Table 2 also displays the final size of the ACP device selected and the need for a change in size, which was required in only 5 patients (a smaller device was required in 4 patients and a larger device in 1). The mean size of the device was 22.06 (3.36) mm (range: 16-28).
Variables of the Intervention and Measurements.
| Intervention time, min | 92.54±38.41 |
| Radioscopy time, min | 21.54±12.87 |
| Contrast volume, mL | 304.14±208.27 |
| Successful implantation | 97.14% (34/35) |
| Cardiac complications | 0% (1 arteriovenous fistula) |
| Need to change the size of the device | 14.28% (5/35); 4 to a smaller size and 1 to a larger size |
| Measurements of LAA neck by TEE, mm | 19.65±3.05 |
| Measurements of LAA neck by angiography, mm | 19.82±3.28 |
| Measurements of the SI axis of the LAA neck by CARTO-CT, mm | 20.40±3.46 |
| Measurements of the AP axis of the LAA neck by CARTO-CT, mm | 17.80±2.95 |
| Average device size, mm | 22.06±3.36 |
AP, anterior-posterior; CT, computed tomography; LAA, left atrial appendage; SI, superior-inferior; TEE, transesophageal echocardiography.
Values are expressed as mean±standard deviation, unless otherwise indicated.
The success of the implantation was 97.14%; the device could not be implanted in only 1 patient. There were no cardiac complications and a vascular complication occurred in only 1 patient: a punctured artery resulting in arteriovenous fistula despite prolonged compression.
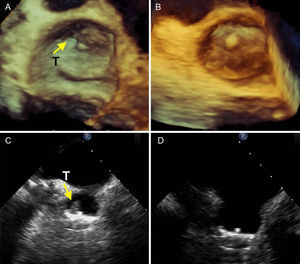
Events During the 1-year Follow-upTable 3 summarizes the results of the follow-up. There were 3 noncardiac deaths in patients aged 85, 83 and 81 years, caused by obstruction and intestinal perforation, intestinal adenocarcinoma, and pneumonia, which led to hospital admission and death. There was severe bleeding in 1 patient with bleeding polyps. Clopidogrel was suspended and only acetylsalicylic acid was maintained, the patient continued treatment in the Gastrointestinal Unit and progressed well. There was 1 mild episode of epistaxis in a patient that forgot to stop the clopidogrel treatment. Thrombi were found in the device in 5 patients (14.28%). One thrombus developed in the first week (the patient who developed a fistula), and antiplatelet treatment was suspended before the vascular intervention. Afterwards, the antiplatelet and enoxaparin treatment was set for 2 weeks. The thrombus disappeared without clinical events. Two other thrombi developed during the second and third month and disappeared 2 weeks after enoxaparin was added. We found images of a 14mm thrombus in the third month in 1 patient that had undergone cardioversion for atrial flutter before discharge, but the patient did not receive enoxaparin due to a fall and facial hematoma. This was the only patient who had a typical transient ischemic stroke (amaurosis fugax) but recovered completely. This patient was admitted for treatment with intravenous heparin, and the thrombus reduced progressively. The fifth case was a laminar mural thrombus in the sixth month which disappeared after 2 weeks of enoxaparin therapy (Fig. 3). We only documented 3 minor periprosthetic leaks in the posterior-superior area, without significant parameters, and minimal shunts of small atrial septal communications in 6 patients, which disappeared during follow-up evaluations. In one patient with a double septum, where a prothrombotic smoke effect developed, we decided to close the foramen ovale with a device. The patient had this problem prior to the intervention.
Clinical Follow-up and Echocardiography Results.
| Time of follow-up, months | 21.14±10.09 |
| Thrombus in TEE | 5 (14.28%) |
| Disappearance of thrombus in TEE | 100% |
| Atrial septal communications | 6 (17.14%) |
| Minimal leaksa | 3 (8.57%) |
| Stroke | 0 (0%) |
| Ischemic strokeb | 1 (2.85%) |
| Hemorrhagesc | 2 (5.7%) |
| Severe | 1 (2.85%) |
| Mild | 1 (2.85%) |
| Cardiac deaths | 0 (0%) |
| Noncardiac deaths | 3 (8.57%) |
TEE, transesophageal echocardiography.
Three Dimensional transesophageal echocardiography (A and B) and 2-dimensional transesophageal images (C and D) with a typical mural thrombus (T) at the upper angle of the device (A and C, arrows) and disappearance of the thrombus (B and D), during follow-up following the addition of dual antiplatelet therapy for 2 weeks with a therapeutic dose of enoxaparin.
The first publications on this technique were with the PLAATO (Percutaneous Left Atrial Appendage Transcatheter Occlusion) device, which was abandoned for a number of reasons. However, in the follow-up of patients with successful implantations, there was a lower percentage of thromboembolic complications than expected according to their CHADS210 score.
Later, the randomized PROTECT AF study, which compared warfarin with the Watchman occlusion device, showed better results for the occluder for more than 1 year, with a significant reduction in hemorrhagic stroke.11 The first ACP12 registry, which showed percentages of efficiency and complications very similar to the PROTECT AF study, was published in Europe, encouraging the continuation of this technique for this subgroup of patients. In these 2 studies of LAA closure, which are the largest published, adverse events decreased by half with greater surgeon experience. In the cohort of patients of the PROTECT AF study, which used the Watchman device (542 patients), adverse events fell from 7.7%-3.7% in the CAP registry, which was continued by some centers which previously participated in the main study but which had gained more experience. In the “Initial European Registry” series (143 patients) with the ACP device, adverse events fell from 7%-3.4% and in the “ACP Postmarket Registry” (145 patients) pericardial effusions fell from 3.5%-2% and ischemic strokes from 2.1%-0%, while the occurrence of device embolization remained at 1.4%. The ACP Italian Registry of 100 patients (G. Santoro; Communication in the congress “Progress in Clinical Pacing”; Rome, 2010) found no device embolizations or ischemic strokes and only 2 pericardial effusions.16,17 The Iberia registry includes cases from Spain and Portugal. There were 213 cases between 2009 and 2011. Of these, 197 were successful (92.5%) and the device could not be implanted in 16 (no data provided through articles and only partially in the communication).18 There were 12 major complications (5.6%) and 6 minor complications (2.81%). The major complications included 3 deaths, 4 device embolizations, 3 resolved cardiac tamponades, 1 stroke, and symptoms of ST segment elevation in the anterior side. Importantly, this registry captures the learning curves of various centers at the same time and, as the case load increased in the different hospitals, these complications clearly decreased.
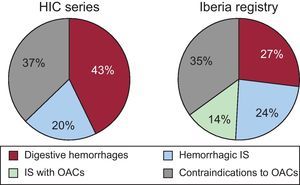
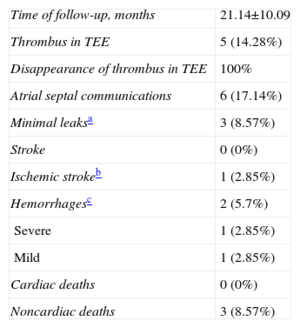
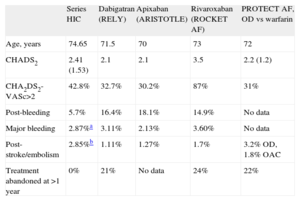
A number of important studies have reported the possible advantages of new OACs over warfarin or acenocoumarol.7–9 Nevertheless, patients that are included in studies such as ours would be excluded from those studies and the PROTECT AF trial for the reasons previously outlined. In theory, this technique does not compete with the new OACs or acenocoumarol, but is a supplement for the 40% of patients who require anticoagulants but who have contraindications for these drugs.6 In our study, the age of the patients was higher than that of published randomized studies. In patients≥80 years, there is a higher percentage of bleeding (13%) compared to published studies based on a lower age group of patients (3%-4%).19 In the RE-LY (Randomized. Evaluation of Long term anticoagulant therapy) study, dabigatran was not found to be more effective than warfarin either in preventing thromboembolism or in reducing hemorrhage in patients≥75 years or in those with a low creatinine clearance, which is frequent in this age group.7 Furthermore, the discontinuation rate is higher with this subgroup of age, reaching 26% in the first year.7,8,19Table 4 shows some of the characteristics of patients in our study compared with those in the main studies. CHADS2 scores of 3 or higher identify patients that could most benefit from a reduction in thromboembolism, but at the same time could bleed more with OACs, including new OACs. Irrespective of whether warfarin or dabigatran was used, these patients had a 2%-3% annual risk for thromboembolism, almost 5% for new major bleeding and almost a 6% risk of death.20 Thus, these patients would obtain maximum benefit from closure of the appendage. It is established that a HAS-BLED score of≥3 represents a high risk of bleeding, with major hemorrhage rates of >4% per year21 and, as expected due to the patients’ characteristics, our study sample had a high HAS-BLED score (4.42 [1.43]). Figure 4 shows the clinical indications for LAA closure in our study and compares them with those in the Spanish registry. Digestive hemorrhages accounted for 43% of the indications (a figure that is slightly higher than that of the Spanish registry data, but is in line with numbers of digestive hemorrhages in patients on OACs),22 followed by patients unable to take acenocoumarol and patients with hemorrhagic stroke caused by acenocoumarol.
Comparison of Some Data of Clinical Interest Between the First Studies With New Oral Anticoagulants and Devices and our Study.
| Series HIC | Dabigatran (RELY) | Apixaban (ARISTOTLE) | Rivaroxaban (ROCKET AF) | PROTECT AF, OD vs warfarin | |
| Age, years | 74.65 | 71.5 | 70 | 73 | 72 |
| CHADS2 | 2.41 (1.53) | 2.1 | 2.1 | 3.5 | 2.2 (1.2) |
| CHA2DS2-VASc>2 | 42.8% | 32.7% | 30.2% | 87% | 31% |
| Post-bleeding | 5.7% | 16.4% | 18.1% | 14.9% | No data |
| Major bleeding | 2.87%a | 3.11% | 2.13% | 3.60% | No data |
| Post-stroke/embolism | 2.85%b | 1.11% | 1.27% | 1.7% | 3.2% OD, 1.8% OAC |
| Treatment abandoned at >1 year | 0% | 21% | No data | 24% | 22% |
ARISTOTLE, Apixaban for Reduction In STroke and Other ThromboemboLic Events in atrial fibrillation; HIC, Hospital Infanta Cristina; OAC, oral anticoagulants; OD, occusion device; PLAATO, Percutaneous Left Atrial Appendage Transcatheter Occlusion; PROTECT AF, PROTECTion in patients with Atrial Fibrillation; RE-LY, Randomized Evaluation of Long term anticoagulant therapY; ROCKET AF, Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonist for the prevention of stroke and Embolism Trial in Atrial Fibrillation.
In patients that could not take acenocoumarol and only for dual antiplatelet therapy in the first months.
In patients who could not take acenocoumarol and only for dual antiplatelet therapy in the first months, where the only case was a transient ischemic attack without sequelae (in Percutaneous Left Atrial Appendage Transcatheter Occlusion 3.8% of strokes vs expected 6.6% according to aCHADS2 score of 2.5).
Indications for the closure of the left atrial appendage in the national registry and in our study (HIC). Contraindications to oral anticoagulants were taken from the clinical history (Table 1). HIC, Hospital Infanta Cristina; IS, ischemic stroke; OAC, oral anticoagulants.
Pending larger follow-up studies, the few thrombotic events which can occur in patients with the device can reasonably be expected to decrease or disappear once the device is integrated within the endothelium after 6 months, as well as the risk of hemorrhage due to dual antiplatelet therapy,22 which is limited to the first 3-6 months.
Due to our strict protocol in the search for thrombi formation in the device, we could observe their development at any time between the first day and the sixth month, but none developed after that period according to checks carried out at 12 months. Studies with less strict protocols reported lower incidence rates, including the absence of thrombi.23 Our protocol for thrombi detection could be considered highly exhaustive but we believe that it is appropriate, at least until the period when thrombi develop is better identified. Except for situations which require the suspension of antiplatelet therapy, monitoring for thrombi should be undertaken at 1, 3 and 6 months. The identification of thrombi is highly important and they respond very well to enoxaparin, probably because they are in an early stage of development during re-endothelialization.24 Although the smoke effect and the size of the left atrium could have influenced our study, there were no clear predictors upon analysis of these aspects.
Finally, satisfactory selection of the size of the device is important. The measurements obtained with intraoperative TEE were slightly lower than angiographic measurements, and the latter were slightly lower than those of the SI axis obtained by CARTO–CT/MRI, the measurement of the short axis being the smallest of the 4 measurements (Table 2). The use of a third measuring technique with MRI or CT could have influenced the finding that the need to change the occlusion device in our study was acceptably low (14.28%), compared with the average in other studies (17%-24%).12,25Figure 5 shows a rate of change in the Iberian Registry of 20%, usually, although not always, changing from a smaller to larger size. In our study, however, it was more frequent to change from a larger to a smaller size, possibly influenced by the decisive weight given to the major axis (SI) of the ostium in the CARTO-CT. This choice was very effective in general (the measurement of the SI axis in the CARTO-CT was accurate in the selection of the correct size in 76.6%, angiography in 66.6%, TEE in 46.6% and the short axis or the anteroposterior view of the CARTO-CT in 26.6%) but in some cases we had to change to a larger device, particularly when there were large ostium measurements (in these cases, angiography was more reliable). The use of these techniques as the ‘gold standard’ has been suggested in some interesting studies, comparing them with other techniques such as 3D echocardiography. However, in these studies, these techniques were not followed by implantation of the device in their patients, which is a limitation.26–30 This third technique is not necessary in most patients, and more in-depth research is required, although it could be used when a device cannot be inserted at the first attempt. Our group observed that a subtype of appendages with low exits and closer to the vestibule of the mitral valve were more complex and could require a more delicate strategy.31
LimitationsIn this study, we did not include the data from the 3D echocardiograms as we only made use of this technique for the final 14 patients. However, 3D echocardiography did not influence changes in the decisions of the measurements that we present, although it was of use during the procedure to see the relationship between adjacent structures and the shape of the appendage.
Our study had a small sample size and therefore clinical efficiency cannot be established, but it is one of the broadest single-center studies, given the slow recruitment of patients. In addition, this study shows the reality of patients in the main hospitals of Spain, and possibly, in the rest of Europe. Procedure and radioscopy times could be slightly above the average, which could be related to the fact that our center is a referral center for learning this technique. To evaluate the clinical efficiency of the technique in patients at high risk of a thromboembolism or hemorrhagic complications, larger studies with a longer follow-up period are necessary.
ConclusionsClosure of the LAA can be a useful alternative to treatment with OACs in patients with nonvalvular AF but is essentially a complementary option for patients who cannot receive OACs due to hemorrhagic complications or recent stroke which contraindicate OAC use. There is a low rate of complications during implantation and very satisfactory results in the clinical follow-up, if the process is carried out by surgeons with experience in this technique and the patients’ risk profile for thromboembolism and hemorrhage is considered. As surgical experience increases, the number of complications in distinct series will undoubtedly decrease, which will consolidate the indications for this technique.
Conflicts of interestNone declared.