Imaging techniques are essential in the clinical evaluation of patients with a myocardial infarction. They are of value for both initial assessment of the ischemic injury and for detection of the subgroup of patients at higher risk of developing cardiovascular events during follow-up. Echocardiography remains the technique of choice for the initial evaluation, owing to its bedside capability to determine strong predictors, such as ventricular volumes, global and regional systolic function, and valvular regurgitation. New techniques for evaluating ventricular mechanics, mainly assessment of ventricular deformation, are revealing important aspects of post-infarction ventricular adaptation. The main alternative to echocardiography is cardiac magnetic resonance imaging. This technique is highly accurate for determining ventricular volumes and ventricular function and has the additional advantage of being able to characterize the myocardium and demonstrate changes associated with the ischemic insult such as necrosis/fibrosis, edema, microvascular obstruction, and intramyocardial hemorrhage. These features not only allow detection and quantification of the infarct size, but also reveal additional characteristics of the scar tissue with prognostic value.
Keywords
The incidence of acute coronary syndrome in Spain remains high, with around 116 000 new events in 2013, and it is expected to increase in the coming decades.1 Imaging techniques are of great value for establishing a prompt diagnosis in myocardial infarction and for prognostic stratification, enabling identification of patient subgroups at a higher risk of complications during their clinical course. Determination of systolic function and ventricular volumes is the cornerstone for predicting events following a myocardial infarction. To evaluate these parameters, transthoracic echocardiography (TTE) remains the test of choice because of its speed and availability, and cardiac magnetic resonance (CMR) is being increasingly used owing to its excellent reproducibility and accuracy. Alternative modalities, such as nuclear techniques and multidetector computed tomography (MDCT), are also available for this purpose. Another important prognostic factor is the size of the infarct, which can be measured with TTE, nuclear techniques, MDCT or, more accurately, with CMR. In addition, capabilities related to myocardial characterization such as determination of the presence of edema, intramyocardial bleeding, and microvascular obstruction by CMR have emerged over the last few years and have shown prognostic relevance.
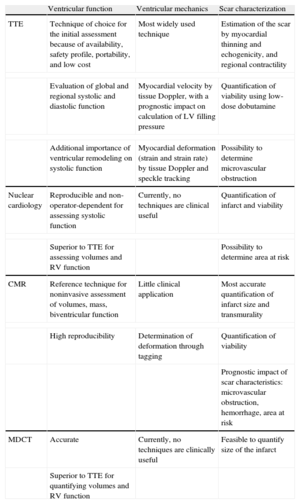
The aim of this review is to update evidence on the current use of imaging techniques, particularly CMR, to assess ventricular function and the myocardial scar following infarction (Table). Detailed coverage of residual ischemia detection, another important aspect in post-infarction risk stratification, is beyond the scope of this article.
Role of Imaging Techniques in Assessment Following Myocardial Infarction
| Ventricular function | Ventricular mechanics | Scar characterization | |
| TTE | Technique of choice for the initial assessment because of availability, safety profile, portability, and low cost | Most widely used technique | Estimation of the scar by myocardial thinning and echogenicity, and regional contractility |
| Evaluation of global and regional systolic and diastolic function | Myocardial velocity by tissue Doppler, with a prognostic impact on calculation of LV filling pressure | Quantification of viability using low-dose dobutamine | |
| Additional importance of ventricular remodeling on systolic function | Myocardial deformation (strain and strain rate) by tissue Doppler and speckle tracking | Possibility to determine microvascular obstruction | |
| Nuclear cardiology | Reproducible and non-operator-dependent for assessing systolic function | Currently, no techniques are clinical useful | Quantification of infarct and viability |
| Superior to TTE for assessing volumes and RV function | Possibility to determine area at risk | ||
| CMR | Reference technique for noninvasive assessment of volumes, mass, biventricular function | Little clinical application | Most accurate quantification of infarct size and transmurality |
| High reproducibility | Determination of deformation through tagging | Quantification of viability | |
| Prognostic impact of scar characteristics: microvascular obstruction, hemorrhage, area at risk | |||
| MDCT | Accurate | Currently, no techniques are clinically useful | Feasible to quantify size of the infarct |
| Superior to TTE for quantifying volumes and RV function | |||
CMR, cardiac magnetic resonance; LV, left ventricle; MDCT, multidetector computed tomography; RV, right ventricle; TTE, transthoracic echocardiography.
The left ventricular ejection fraction (LVEF), a powerful predictor of cardiovascular events, is the most widely used parameter in clinical practice to assess the changes occurring in cardiac function after a myocardial infarction.2 However, it is important to keep in mind that both global and regional systolic function can be misleading in the acute phase due to myocardial stunning in noninfarcted segments or compensatory hypercontractility at distant sites. Transthoracic echocardiography is the technique of choice for first-line post-infarction evaluation of ventricular volumes and systolic function.3 Simpson's modified biplanar method is currently recommended, in particular for patients with changes in regional contractility.4 Evaluation of regional contractile function is useful for establishing the diagnosis of an acute coronary syndrome and also has prognostic implications. The wall motion score index, which reflects the extent of contractile dysfunction by estimating the motion index of the 17 left ventricular (LV) segments, has proven to be a predictor of mortality and hospitalization for heart failure following an infarction, regardless of the LVEF.5
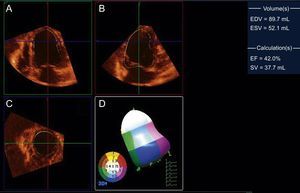
Although TTE is widely available, 10% of studies have been estimated to have image quality limitations that hamper interpretation of their findings.6 The use of echocardiographic contrast agents improves visualization of the endocardial border and accuracy in the evaluation of global and regional systolic function.7,8 In the past, implementation of these agents in acute coronary syndromes was considered contraindicated, but current recommendations allow their use in this context with proper monitoring.9 Another developing echocardiographic technique that has shown increasing clinical utility in daily practice is 3-dimensional echocardiography, particularly in real time. Although a good acoustic window is still required to obtain high-quality studies, this technique enables reformatting of the 3-dimensional data set in any imaging plane and avoids geometric assumptions (Figure 1). In LVEF assessment, 3-dimensional echocardiography has shown excellent correlation and agreement with CMR and better interobserver and intraobserver reproducibility than conventional TTE.10 These findings have been confirmed in infarction patients,11 including in serial follow-up of LV function.12 Nonetheless, it remains controversial whether 3-dimensional echocardiography is also superior to conventional TTE for evaluating regional abnormalities (Figure 1), although administration of contrast agents may improve the diagnostic yield.13
Three-dimensional transthoracic echocardiography of the left ventricle in an experimental infarction model, reformatted in 4-chamber (A), 2-chamber (B), and short-axis (C) views, and analysis of regional contractility (D), which shows apical hypokinesia (in white in the 3-dimensional model). The left ventricular volumes and left-ventricular ejection fraction are shown in the right column. EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; SV, stroke volume. Courtesy of Drs. C. Santos-Gallego and J. Badimón.
The most widely used alternative to TTE is CMR, which is currently considered the noninvasive reference technique for measuring the biventricular ejection fraction.14 This noninvasive technique is highly reproducible, a characteristic that is of particular interest in research because it enables reliable study of smaller samples.15 Furthermore, CMR can accurately evaluate regional contractile function abnormalities.16 Other advantages of this technique include an absence of ionizing radiation exposure and the need for contrast agents, although it is less widely available and more costly than TTE. The LVEF and ventricular volumes can be obtained using synchronized single-photon emission computed tomography (SPECT) studies, which have well-characterized prognostic value.17 This technique is not operator-dependent and does not rely on geometric assumptions, but it does use ionizing radiation, and consequently is not commonly used to measure LV function as the only indication. For this same reason, MDCT is rarely used to determine LV volumes and function exclusively, even though it provides highly accurate measurements.18
Left Ventricular Diastolic FunctionIn conjunction with systolic function, noninvasive assessment of diastolic function by Doppler TTE study of the transmitral flow pattern to estimate LV filling pressure is an important element in the evaluation of an infarction patient. The impact of a restrictive filling pattern on mortality and the incidence of heart failure was soon evident with the use of this technique19 and was subsequently confirmed.20 A recent meta-analysis reported that the presence of a restrictive pattern (defined as an elevated E/A ratio with a deceleration time of E<140ms) identifies a more than 2-fold higher risk of death after adjusting for age, sex, and LVEF.21
The development of tissue Doppler has enabled concomitant measurement of myocardial motion velocities and early diastolic mitral annular velocity (e’) to provide noninvasive estimation of LV filling pressure. In this regard, Hillis et al22 demonstrated that an E/e’ value > 15 in the initial evaluation following infarction was associated with a 5-fold greater risk of death during follow-up. This finding was further supported when the E/e’ was shown to identify a subgroup of patients with greater mortality even in the presence of elevated B-type natriuretic protein values.23 These data indicate that in addition to systolic function, myocardial stiffness and relaxation abnormalities play an additional role to systolic function.
The main limitation of Doppler derives from its dependence on hemodynamic conditions; hence, it would be of interest to use a more stable and reproducible parameter. For this reason, the prognostic relevance of left atrial size has been evaluated. An indexed volume > 32mL/m2 is a powerful predictor of medium-term24 and long-term25 mortality following infarction, regardless of the presence of mitral regurgitation or atrial fibrillation.
Left Ventricular Dilatation and RemodelingEarly experimental studies26 were quick to establish the importance of ventricular remodeling following an ischemic injury. This phenomenon is defined as a change in the ventricular architecture, consisting of a volume increase and changes in the morphology of the cardiac chambers, occurring following ischemic injury. The initial beneficial effect of LV dilatation to maintain the volume pumped is offset in the long-term by a deleterious effect, likely mediated by neuroendocrine stimulation triggered by increased wall stress. In a meta-analysis by Kramer et al27 analyzing interventional studies in patients with systolic dysfunction and heart failure of ischemic and nonischemic origin, a correlation was found between the changes in ventricular remodeling or the LVEF (measured by different techniques) and the effect on mortality. This could indicate that the benefit of these treatments may be mediated, at least in part, by their effect on these ventricular parameters. Several clinical trials28,29 have shown that the study of ventricular remodeling, represented by the LV end-diastolic and end-systolic volumes, provides further information in addition to LVEF for predicting major events following a myocardial infarction. LV sphericity, another remodeling parameter, has also proven to be a predictor of mortality, even after adjustment for the LVEF and LV end-systolic volume.30 The reference technique for measuring ventricular volumes is, once again, CMR.14 Although 3 -dimensional TTE shows better agreement with CMR than conventional TTE, it can also underestimate ventricular volumes.10
In addition to ventricular dilatation, ventricular hypertrophy also has prognostic relevance. In an echocardiographic substudy of the VALIANT trial,31 the pattern of ventricular remodeling, defined by the indexed LV mass and relative wall thickness, correlated with the incidence of post-infarction major cardiovascular events. Concentric remodeling, eccentric hypertrophy, and concentric hypertrophy were independently associated with a gradual increase in cardiovascular death, reinfarction, heart failure, stroke, and sudden death during follow-up compared with an absence of LV remodeling.
Right Ventricular FunctionRight ventricular (RV) infarction is associated with an increased risk of in-hospital complications and death.32 In autopsy studies, up to 50% of myocardial infarctions show RV involvement;33 however, clinical detection of these abnormalities is less frequent, and TTE has considerable limitations for evaluating RV systolic function.34 Three-dimensional echocardiography35 is superior to conventional TTE for assessing RV infarction and provides optimal identification of patients with right systolic dysfunction. A CMR study36 has confirmed that RV involvement is an independent prognostic marker, regardless of LVEF: an RV ejection fraction < 40% is associated with a 27% absolute increase in the risk of major cardiovascular events at 4 years’ follow-up.
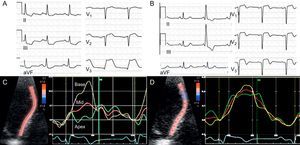
Evaluation of Ventricular MechanicsThe last few years have witnessed significant advances in the study of cardiac mechanics by noninvasive techniques, whose clinical applications are under development. The most important of these is tissue Doppler echocardiography, and in particular, speckle tracking. These modalities enable quantitative determination of the regional myocardial velocity, percentage of strain, and strain rate in radial, longitudinal, and circumferential directions.37 In both animal models38,39 and clinical studies,40,41 reductions in these parameters have enabled identification of regional contractility abnormalities. Because myocardial thickening greatly depends on the subendocardial myocardial fibers, a pattern showing a loss of regional radial function with relative preservation from longitudinal and, particularly, circumferential deformation has been proposed as a marker of nontransmural infarction.42,43 Measurement of global myocardial deformation using these techniques has also been proven to have clinical value. In a study of 659 post-infarction patients, preservation of the global longitudinal strain and strain rate was a better predictor of survival than the LVEF or wall motion score index.44
Myocardial deformation can also be determined using CMR. Myocardial tagging is the most extensively used technique, and many authors consider it the reference method for this purpose.40 A straight-line grid of magnetic saturation signal intensity (tags) is applied to the myocardium and maintained during the cardiac cycle to visualize LV deformation, which can then be quantified by specific post-processing software. An experimental animal model45 showed that differences in contractile function between regions adjacent to the infarction and distal regions persisted during the process of ventricular remodeling. These findings were later corroborated in a clinical study.46 In other CMR sequences, the myocardial signal intensity is proportional to the myocardial velocity (phase contrast), displacement (displacement encoding with stimulated echoes, and deformation (strain-encoded imaging); this enables simpler post-processing and has proven useful in patients with myocardial infarction.47–49 Preliminary studies have also demonstrated the possibility of analyzing myocardial deformation with MDCT50 and SPECT.51
Echocardiographic techniques for the study of myocardial deformation have also been used to evaluate LV torsion,52,53 which has been correlated with the intraventricular pressure gradient.54 In patients with myocardial infarction, a reduction in subendocardial twist has been observed that is directly related to the extent of the infarction.55 Following anterolateral infarction, CMR has also shown smaller apical rotation, and a delay and prolongation of diastolic untwisting, which are completely lost in ventricular aneurysms.56
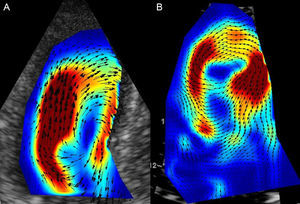
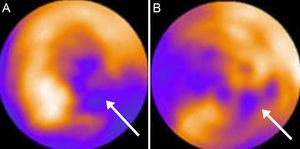
Finally, evaluation of intraventricular fluid dynamics during the cardiac cycle and characterization of vortex by contrast-enhanced TTE (Figure 2) has generated considerable recent interest. Nucifora et al57 have reported that abnormal myocardial vortex parameters show a correlation (although weak) with diastolic function in patients with a myocardial infarction. Nonetheless, these parameters are still a long way from being incorporated into clinical practice.
CARACTERIZATION OF MYOCARDIAL INFARCTIONGeneral ConsiderationsThe size of the infarct has been classically assessed using basic clinical tools such as electrocardiography, enzymatic markers of myocardial injury, and global or regional contractile function abnormalities. Additional characterization of the size of the scar and myocardial viability can be carried out with fully validated techniques, such as dobutamine TTE, SPECT, and positron emission tomography. In recent years, however, CMR has emerged as a multipurpose test that not only enables evaluation of contractility, but also provides better-quality tissue characterization than TTE without using ionizing radiation. Preliminary studies have also reported the possibility of characterizing the infarct with MDCT. This section focusses on the role of imaging techniques in the characterization of the infarct scar, with special emphasis on CMR.
Detection and Quantification of the InfarctIf a significant reduction in coronary flow occurring during an acute coronary syndrome lasts for a sufficient amount of time, the cardiomyocytes undergo gradual necrosis, progressing from the subendocardium to the subepicardium, which is later replaced by myocardial fibrosis. Initially, the presence of interstitial edema in the necrotic territory may reach twice the apparent size of the infarct. Thereafter, gradual replacement of necrotic tissue with fibrosis may reduce the volume by 25%.58 Furthermore, compensatory hypertrophy occurring in the distal segments can change the size of the infarct relative to the total LV mass.59 At around week 6, the infarct size is considered relatively stable,60 which should be taken into account when interpreting the results of imaging studies.
These histological changes can be manifested as an absence of radioisotope detection in the infarcted area by nuclear techniques that have been fully validated for quantifying infarct size, in particular, SPECT.61 More recently, infarct size has been visualized with CMR, through a phenomenon known as late gadolinium enhancement (LGE); that is, an accumulation of contrast in the infarcted regions minutes after contrast is administered.5–30 This is due to a buildup of gadolinium chelates (strictly extracellular agents) within the intracellular space during the acute phase, caused by a loss of integrity of the cardiomyocyte membranes, and within the interstitial space during the chronic phase, caused by expansion of the space during the development of fibrosis.62 The same phenomenon occurs with iodinated contrast agents, and preliminary studies have demonstrated the possibility of studying the infarct with MDCT.63
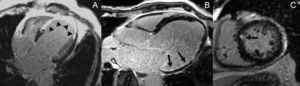
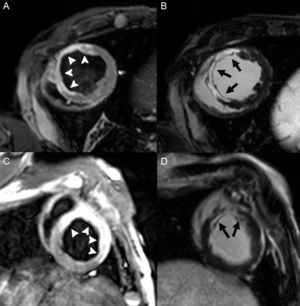
The capability of of this technique to determine the size of the infarct has been extensively validated in animal models.64 The excellent spatial resolution of LGE enables determination of the degree of transmural infarction (Figure 3). This test has proven to be more sensitive than nuclear medicine tests for detecting subendocardial infarction,65,66 and is able to detect infarcts as small as 1g67 (Figure 3). For these reasons, LGE-CMR has become the reference clinical technique for evaluating the myocardial scar. It has shown that the prevalence of subclinical infarction is more common than was previously believed and is associated with a poorer prognosis.68,69 Moreover, LGE enables detection of specific complications, such as RV infarction (also associated with a poorer prognosis),70 papillary muscle involvement,71 and ventricular thrombus72 (Figure 4).
Quantification of infarct size using LGE can be carried out by various techniques. The visual method consists of applying a 5-point scale to each of the 17 LV segments, as follows: 0, no hyperenhancement; 1, 1% to 25% of thickness of the segment; 2, 26% to 50%; 3, 51% to 75%, and 4, 76% to 100%. Infarct size as a percentage of LV myocardium can then be calculated by summing the segment scores and dividing by the number of enhancing segments.73 Various semiautomated quantitative methods for segmentation of the infarct are available, based on application of an intensity threshold as related to the viable distal myocardium. The best criterion seems to be full width at half maximum,74 although there is still controversy around this point. Lastly, the absolute or relative mass of the infarct can be delimited manually. This method is considered accurate and of similar prognostic value if the analysis is performed by expert observers.75 Several studies have confirmed the prognostic relevance of infarct size determined by CMR as a better predictor of major cardiovascular events and mortality than systolic function or ventricular volumes when determined in the acute phase,76 and particularly, in the late phase following an ischemic event.77 The degree of transmurality of the scar has also been identified as a prognostic marker in some studies.78
One of the potential applications of characterizing the scar by LGE is to predict malignant ventricular arrhythmias. The importance of the extent of infarction in the development of ventricular arrhythmia has been investigated retrospectively in patients with coronary disease; the size of the area of LGE was found to be an independent predictor of appropriate defibrillator shocks79 and mortality.80 A later prospective study in patients with a primary indication for defibrillator implantation according to the MADIT (Multicenter Automatic Defibrillator Implantation Trial) criteria confirmed that relative infarct transmurality is an independent predictor (odds ratio=22.1) of appropriate defibrillator shocks and sudden death.81 The heterogeneity of the scar can be determined by mapping the variations in signal intensity. Heterogeneous areas correspond to the presence of conducting channels, which are the substrate of ventricular arrhythmia after an infarction.82 In addition, the preinfarct, or grey area, has been identified, which contains both necrotic and viable tissue. This has been associated with cardiovascular death,83 inducibility of ventricular arrhythmias,84 and appropriate defibrillator therapy in both primary and secondary prevention.85 However, not all the related studies have found an independent value of this parameter with respect to the total size of the infarct.86
Myocardial ViabilityLow-dose dobutamine stress echocardiography and SPECT are extensively validated techniques commonly used in daily practice.87 Positron emission tomography, which mainly detects viability as a mismatch between myocardial perfusion and metabolism (Figure 5), is a highly sensitive technique for predicting functional recovery, although it is less widely available in the clinical setting.87 Microcirculation integrity demonstrated with contrast-enhanced TTE has also been associated with residual viability.88 Similarly, preservation of the parameters indicating global and regional myocardial deformation is inversely related to transmurality and the extension of the infarct,89,90 and has been proposed as a marker of myocardial viability (Figure 6). Improvements in the regional strain and strain rate with low dobutamine doses also predict viability91 and have shown better diagnostic performance than visual evaluation.92 On CMR, viability can be detected by the increase of contractile function with dobutamine or, more often, by the degree of scar transmurality on LGE. The degree of transmurality is inversely related to the probability that contractility will recover following revascularization in the case of myocardial hibernation93 or spontaneously in the case of myocardial stunning.94 In fact, a recent study has questioned the classic concept that myocardial thinning necessarily indicates inviability by demonstrating improvements in thickness and contractility following revascularization when LGE transmurality is < 50%.95 in general, LGE has a higher sensitivity (95%) and negative predictive value (90%) for predicting segmental functional recovery, while low-dose dobutamine stress echocardiography shows greater specificity (91%) and a higher positive predictive value (93%).96
Radial strain measured by speckle tracking before and after reperfusion in a patient with acute infarction. During the coronary occlusion demonstrated by ST segment elevation (A) there is a progressive decrease of radial systolic strain from the base to the apex (C). Over follow-up, despite the presence of Q waves on electrocardiography (B), regional function has recovered (D), which indicates viability. Courtesy of Drs. M. Amaki and P. Sengupta.
A substudy of the prospective, randomized STICH97 trial has recently questioned the clinical significance of myocardial viability for deciding whether revascularization is needed in patients with systolic dysfunction of ischemic etiology. Viability was not identified as an independent predictor of 5-year mortality, nor did it show an interaction with the strategy of revascularization in prognosis. Of note, one of the limitations of this study was that less than half the patients initially recruited were included in this subanalysis and that the indication for a viability study was at the discretion of the attending physician. In addition, the study was performed with SPECT and/or dobutamine TTE. In a prospective study of 144 patients,98 the presence of viability on CMR in the absence of complete revascularization was associated with an almost 5-fold increase in mortality, whereas in the absence of significant viability, revascularization did not improve prognosis. Nonetheless, it is questionable whether the use of CMR (or positron emission tomography) would have led to changes in the STICH results.99
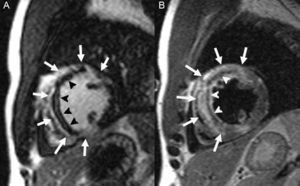
Assessment of the Area at Risk and Myocardial SalvageStandard, noninvasive techniques for evaluating the myocardial area at risk during coronary occlusion require injection of radioisotopes100 or echocardiographic contrast88 before reperfusion. On another note, CMR has been used to detect myocardial edema in the acute and subacute phases of infarction with specific T2-weighted sequences that enable differentiation between acute and chronic infarction101 and identification of patient subgroups at higher risk during an acute coronary syndrome.102 The edematous areas evaluated days after an ischemic event correspond to the area at risk, as has been validated with radioactive microspheres in animal models,103 and by angiographic scores104 and SPECT,105 considered by many the reference technique, in humans. The area of myocardium at risk defined by T2-weighted sequences is always larger than the size of the infarct determined by LGE, and the myocardial salvage index is calculated as the difference between the 2 values (Figure 7). This parameter has been identified as an independent predictor of mortality and major cardiovascular events at 6 months106 and 18107 months following infarction. Furthermore, determination of the size of the infarct, area at risk, and myocardial salvage is extremely useful in the evaluation of new cardioprotective therapies.108
Calculation of myocardial salvage by demonstration of edema (A and C, arrowheads) and late gadolinium enhancement (B and D, arrows) in an experimental infarction model. The upper row shows an animal in which the areas at risk (edema) and the infarcted areas (late gadolinium enhancement) are similar, indicating little myocardial salvage. The lower row shows another animal, in which the area at risk is much larger than the infarction, which indicates extensive myocardial salvage. Courtesy of Drs. C. Santos-Gallego and J. Badimón.
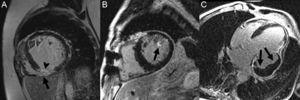
In addition to infarct size the infarct, other aspects related to characterization of the scar also have a prognostic impact. Injury to the myocardial microcirculation can ensue in relation to severe ischemic injury or the reperfusion carried out. This occurrence, which is known as microvascular obstruction or no-reflow phenomenon, has been classically evaluated with contrast-enhanced TTE, which demonstrates myocardial perfusion abnormalities.88 More recently, this assessment has been done with CMR, in which the microvascular obstruction is visualized as a persistent perfusion defect in first-pass sequences or as an unenhanced area in the center of the scar on LGE109 (Figure 8A). Microvascular obstruction is a powerful independent predictor of a permanent inability to recover contractility, adverse remodeling, and subsequent cardiovascular events.110,111
In addition, microvascular ischemic injury can cause extravasation of blood and hemorrhage in the infarct, particularly after reperfusion. On CMR this is seen as hypointense areas on T2-weighted sequences,112 or more specifically, on T2*-weighted sequences,113 owing to the presence of products of hemoglobin breakdown (Figure 8B). Hemorrhage in the infarct area has also been associated with larger infarct size, adverse ventricular remodeling, and an absence of improvement in contractile function.112,114
CONFLICT OF INTERESTSNone declared.
Section sponsored by Laboratory AstraZeneca.