Keywords
INTRODUCTION
The frequency of stroke and its associated mortality have decreased over the last 25 years in most developed nations. However, cerebrovascular disease is the third leading cause of death in Spain and the most frequent cause of death among women.1 The prevalence of the disease is nearly 7000 cases per 100 000 persons older than 64 years.2 The prevention and correct treatment of patients with stroke and its complications thus have important repercussions, not only for the patients themselves but also for their families, health services and society in general.
BASIC CONCEPTS OF STROKE FOR THE CARDIOLOGIST
Generally speaking, cerebrovascular diseases are caused by damage to the cerebral circulation resulting in a transitory or permanent change in brain function. These disorders, which can be grouped according to their overall pathological mechanism, have different clinical repercussions (Figure 1).
Fig. 1. Pathological classification of stroke. TIA: transient ischemic attack. Adapted from Diez Tejedor E, et al.4
Ischemic stroke
Ischemic stroke is the result of a quantitative or qualitative reduction in blood supply to the brain. Ischemic stroke in a localized area of the brain is usually divided into two broad groups:3,4
1. Transient ischemic attack (TIA). This is a focal or monocular dysfunction of the brain secondary to a thrombotic event or arterial embolism, with symptoms lasting less than 24 hours. There are four large subgroups of TIA, depending on the symptoms: retinal, lacunar, hemispheric and atypical. A topographical classification of the vascular territory affected makes it possible to distinguish between carotid, vertebrobasilar or indeterminate involvement.
2. Cerebral infarction. This involves a change in blood supply to a territory of the brain, and symptoms last longer than 24 hours. There are five subtypes with important clinical implications:
- Atherothrombotic infarction: this is usually a medium-sized or large infarction with a cortical or subcortical topography and arising in carotid or vertebrobasilar vessels. A basic indicator of atherothrombotic infarction is that the patient suffers arteriosclerosis; this results in symptoms of peripheral vascular disease, alterations in the coronary vessels, and either occlusion or stenosis of the cerebral arteries.
- Cardioembolic infarction: this is a medium-sized or large infarction with a cortical topography. The only known cause is emboligenic heart disease.
- Lacunar infarction: this is a small infarction in patients with a history of hypertension or other risk factors. The infarction is localized to the territory of a cerebral perforant artery and is accompanied by lacunar syndrome. This presents as a pure sensory syndrome, ataxic hemiparesis and clumsy hand syndrome, a motor sensory syndrome or pure motor hemiparesis.
- Infarction of unusual cause: this is an infarction in which the first three causes are all ruled out. Its origin is related with numerous systemic diseases, including neoplasia, myeloproliferative syndromes, coagulation disorders, connective tissue disorders and infections. The infarction can be of any size, and be localized cortically or subcortically in a carotid or vertebrobasilar territory.
- Cerebral infarction of indeterminate origin: this is the catch-all term for all those cases not included in the previous groups.
Hemorrhagic stroke
This type of stroke is related with a hemorrhage in the cranial cavity secondary to extravasation from a ruptured blood vessel. Hemorrhagic strokes account for 20%-25% of all strokes. They are most commonly related with hypertension, followed by arteriovenous malformations, aneurysms, use of anticoagulants, hematological disease and use of drugs such as cocaine and alcohol.
IMAGING TECHNIQUES FOR EVALUATION OF PATIENTS WITH STROKE
A patient with suspected stroke undergoes a battery of diagnostic tests to provide a definitive diagnosis and information regarding severity and prognosis, as well as the result of any treatment options (Tables 2 and 3).
Imaging techniques have revolutionized the evaluation of patients with stroke. These techniques can be grouped under three main headings:
1. Neuroimaging techniques.
2. Cardiac imaging techniques: cardiac embolism
3. Doppler echocardiography of the supra-aortic trunk and transcranial Doppler.
Neuroimaging techniques
Evaluation of the cerebral anatomy is fundamental in patients with stroke, not only to establish a topographic diagnosis of the lesion, but also to define the origin, reach a differential diagnosis and distinguish between non-reversible areas and areas with a chance of recovery. Many methods are available to evaluate these patients:
Computerized tomography (CT)
Undoubtedly, CT is the most widely used technique to study patients with stroke; it should be done systematically and as soon as possible. The main advantage of CT is its high specificity for the differential diagnosis between ischemic and hemorrhagic stroke. However, its sensitivity for the early detection of ischemic tissue is low, and up to 60% of patients have a strictly normal cranial CT during the initial hours after a stroke.
Cranial magnetic resonance imaging (MRI)
Conventional MRI offers no real advantages over cranial CT for the detection of cerebral infarction. Bryan5 showed that during the first three hours after an ischemic stroke there is no difference between conventional MRI and CT without contrast. However, the new MRI diffusion techniques seem superior during the early stage of ischemia. Alterations in diffusion can be detected less than one hour after occlusion of the middle cerebral artery.6,7 The sensitivity and specificity of these techniques in acute stroke are almost 100%8,9 and diagnostic errors occur mostly in small lacunar lesions in the brain stem. Modern MRI techniques with injection of paramagnetic contrast agents provide information about the intravascular space and enable the state of cerebral perfusion to be directly and dynamically evaluated. Magnetic resonance perfusion studies afford greater sensitivity in the early detection of ischemia. The combination of diffusion and perfusion studies gives very useful hemodynamic information to evaluate the therapeutic results of fibrinolysis versus neuroprotective agents.
Cardiac imaging techniques: cardiac embolism
Transthoracic echocardiography (TTE), and more especially transesophageal echocardiography (TEE), play a basic role in the search for the cardiac embolism causing the stroke. Their systematic use in the evaluation of patients with stroke has led to the understanding of the actual meaning of old etiologies which were traditionally considered to be the origin of the embolic source, as well as to the recognition of new causes, some with important clinical implications and of great interest to the cardiologist (Table 1). This review will make special mention of those causes of stroke for which echocardiographic study is essential:
Valve calcification
Radiographic techniques were the first to show the presence of valve calcifications in vivo. Fluoroscopy with field amplification is able to disclose calcium deposits on the valves or valvular rings.10 Likewise, CT and cine-CT are highly sensitive techniques for the visualization of calcifications.11 Echocardiography, however, is the technique of choice because it makes it possible to localize the calcium in the aortic sigmoid valves or the mitral valve and ring, as well as providing clear definition of its extent.
Much controversy exists concerning the mechanism by which intracardiac calcium may be the cause of embolism. Embolization of calcified material is unusual, though cases have been reported in the cerebral vascular tree, and in peripheral and even in coronary embolisms.12-15 The embolism is most likely related with platelet activation and fibrin deposits on the irregular surface of the calcium in contact with the bloodstream.16
The classic study by Nair17 found stroke in 10% of the patients with mitral ring calcification and only 2% of 101 age- and sex-matched controls.18 Likewise, in a study of persons older than 80 years of age, 20% of a group of more than 500 patients with mitral ring calcification had had peripheral thromboembolism versus 12% of the control group. In the BAATAF study, after 28 months of follow-up, 7.8% of the patients with calcifications had embolic events, compared with 1.7% of the control group.19 Consideration should be given to associated factors which might introduce errors when evaluating the results. Atrial fibrillation has been shown to be more common in patients with mitral ring calcification, and this could be the real cause of the stroke.20,18 Nevertheless, the Framingham study showed the incidence of stroke to be significantly higher in patients with ring calcification.21 After adjusting for variables such as sex, age, diabetes and atrial fibrillation, the relative risk was twice as high in patients with calcium.
No study has yet shown any therapeutic benefit in patients with mitral ring calcification. Its diagnosis, therefore, should not lead to a change in therapy.
Strands
Strands, which are 5-to-10-mm-long, thin, mobile, filamentous attachments, are usually situated on the atrial surface of the mitral valve, though they may also be detected on the aortic valve, especially on the ventricular surface, and on artificial heart valves. Magarey first described these structures in 1949 after an exhaustive analysis of more than 250 specimens. He defined them as endothelialized fibrin deposits in the area of leaflet coaptation, probably related with increased stress in areas of maximum valvular tension.22
Transesophageal echocardiography is the diagnostic technique of choice. All care should be taken, and the study should be undertaken with multiplanar esophageal imaging to provide an exhaustive study of the valve (Figure 1). Diagnostic problems may occasionally arise in patients with suspected endocarditis; the vegetations are usually thicker and irregular, although it is often not possible to make a definitive diagnosis.
The presence of strands in the left heart has sometimes been related with an increased risk of stroke.23,24 However, long-term follow-up studies of patients with an incidental TEE finding of strands show the four-year probability of stroke to be very low--less than 1%.24 Furthermore, Cohen confirmed that the presence of strands in older patients does not increase the risk of stroke.24 Although strands occasionally disappear after treatment with dipyridamole,25 the requirement for prophylactic therapy in patients with strands and stroke has not yet been established, nor has the most appropriate treatment, i.e., anticoagulation versus antiplatelet agents.
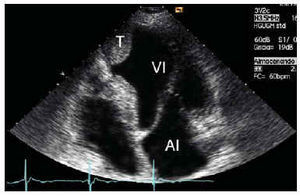
Spontaneous echocardiographic contrast
In 1983 our group first described the finding, with TTE, of intracavitary echogenic images in the left atrium of patients with mitral valve disease.26,27 With the advent of TEE and higher frequency probes (5 MHz), spontaneous contrast has become a much more frequent finding, seen in 30%-70% of patients with mitral stenosis in atrial fibrillation. The technique currently used for the detection and evaluation of spontaneous contrast is TEE, particularly with high frequency probes (Figure 2). The prevalence of spontaneous contrast in the left atrium is 19%-59%, depending on the series and clinical circumstances of the patients.28-31 Factors related with the presence of spontaneous contrast include atrial fibrillation, left ventricular dilatation and reduced left atrial flow.
Fig. 2. Strand on the ventricular surface of the aortic valve (arrows). RV indicates right ventricle; AV, aortic valve.
Spontaneous contrast in the left atrium occurs in 30%-70% of the patients with mitral stenosis, and its frequency is directly related with the severity of stenosis. Spontaneous contrast in the left atrium is found in about 25% of the patients with non-rheumatic atrial fibrillation and in 50% of those with rheumatic atrial fibrillation.28,31 Although the association of spontaneous contrast in the left atrium with atrial flutter is less frequent than its association with atrial fibrillation, its finding on echocardiography is not uncommon in patients with atrial flutter. Irani, for example, reported an incidence of 26%.32 The presence of mitral incompetence appears to be associated with a lower prevalence of spontaneous contrast in the left atrium.33,34
The association in the left atrium of spontaneous contrast and thrombi has been widely reported.26,27,35-37 From 29% to 60% of the patients with spontaneous contrast in the left atrium also have thrombi in this chamber,35,31 and about 80% of the patients with thrombi in the left atrium also have spontaneous contrast in this chamber.32,38 Spontaneous contrast in the left atrium is associated with a greater incidence of stroke and other acute arterial embolisms, especially in the presence of other conditions such as left atrial dilatation, and particularly, atrial fibrillation.39-41 This occurs not only in patients with mitral valve disorders, but is also seen in other situations. Shen et al, used TEE to study 86 patients with dilated cardiomyopathy and reported that 36 (42%) had intracavitary spontaneous contrast, located in the left atrium in 35.42 The presence of spontaneous contrast in this study was related with a greater prevalence of intracavitary thrombi and with a higher incidence of stroke. Leung et al, who used TEE to study 272 patients with non-rheumatic atrial fibrillation, found spontaneous contrast in the left atrium in 161 (59%).43 After 17.5 months of follow-up, the annual incidence of stroke or other type of acute arterial embolism was 12% in patients with spontaneous contrast in the left atrium. Long-term mortality was significantly higher in patients with spontaneous contrast. Indeed, the only independent risk factor for the occurrence of embolic events was the presence of spontaneous contrast in the left atrium.43
Despite the relation between spontaneous contrast in the left atrium, the formation of atrial thrombi and the incidence of systemic thromboembolism, no studies have yet identified those patients in sinus rhythm with spontaneous contrast in the left atrium who might benefit from long-term anticoagulation therapy.
Aortic arch atheromas
The first clear evidence of an association between atheromatous plaques in the aorta and ischemic stroke was provided by Amarenco. In a series of 500 autopsy studies he found ulcerated plaques in 26% of the patients with stroke and only 5% of the control subjects. Because no echocardiographic study had been undertaken prior to death, the correlation between these findings and TTE was unknown. After the introduction of TEE (Figure 3), numerous studies have confirmed that the presence of an atheroma in the aortic arch is associated with a 3-to-9-fold higher risk of stroke.44-47 The greatest increase in risk is seen in patients in whom TEE shows morphologically complex plaques, with superimposed ulcerations and thrombi, which can result in up to a 17-fold increase in risk.48 Results of the SPAF study suggest that once complex plaques are detected, anticoagulation with warfarin to maintain the INR between 2 and 3 is an alternative means of reducing embolic events.49 Greater controversy surrounds the regimen for patients with stroke who have large, non-complicated plaques (>4 mm). Although such patients may benefit from anticoagulation with warfarin,49 the most reasonable option is probably antiplatelet drugs and statins. Surprisingly, no controlled studies have yet defined the real role of antiplatelet agents in the prevention of events in patients with complicated plaques. Stern50 suggested the use of endarterectomy to extract complicated atheromas. However, this procedure is associated with substantial morbidity and mortality, and it should be reserved for highly selected cases.
Fig. 3. Transesophageal echocardiography in a patient with mitral stenosis. The left atrium is full of spontaneous contrast. RA indicates right atrium; MV, mitral valve; LA, left atrium.
Ventricular thrombi
Transthoracic echocardiography is the diagnostic technique of choice for both acute myocardial infarction and for dilated cardiomyopathy; TEE is useful only in exceptional cases. Echocardiographically, a thrombus presents as a space-occupying mass in the ventricular cavity, with greater birefringence than the adjacent myocardium and usually situated in an area of poor contraction (Figure 4).
Fig. 4. Transesophageal echocardiography of the thoracic aorta in a patient with complicated ulcerated aortic plaque.
From 1% to 2.5% of the patients with acute myocardial infarction have a stroke, more than half within one week and the rest within one month. The risk remains relatively high for the first three months, and decreases thereafter. The causes of stroke within the context of infarction are well known. They include localization of the infarction (anterior infarctions are four times as likely to cause thrombi as inferior infarctions), the presence of accompanying atrial fibrillation, and ventricular dysfunction.
Echocardiography can be useful to establish anticoagulation regimens in patients with acute myocardial infarction. In inferior infarctions, anticoagulation should be given if TTE shows the presence of thrombi. In anterior infarctions, anticoagulation should be given until TTE rules out the presence of an apical thrombus. Anticoagulation therapy should not exceed 6 months and the thrombus should be monitored for mobility, protrusion and size. Longer-term anticoagulation therapy is especially beneficial in patients with poor ventricular function. In the SAVE study, each 5% reduction in ejection fraction was associated with an 18% increase in the risk of stroke.51
In patients with dilated cardiomyopathy, the risk of peripheral vascular disease is from 2% to 4% per year, and TTE is again the diagnostic technique of choice to detect it. A clear relation exists between ejection fraction as measured by echocardiography and the risk of embolization. The SOLVD study52 linked this relation especially with female sex. No studies have yet been reported which evaluate the efficacy of anticoagulation in patients with dilated cardiomyopathy. However, if a thrombus is seen in the echocardiographic study, warfarin therapy should be given until serial studies demonstrate that it has disappeared.53 The SAVE study51 showed the usefulness of preventive therapy with aspirin in the absence of echocardiographic evidence of thrombus, in patients who are in sinus rhythm and who have no history of embolization.
Infectious endocarditis
Peripheral embolism is one of the most common complications in patients with infectious endocarditis, although the actual incidence is difficult to establish because the embolism may often be subclinical. Autopsy studies have shown embolization rates in the brain of up to 40%.54 In our series, neurological alterations were the first sign in 33% of all patients,55 although systemic embolization was only seen in 27%. The embolic accident usually occurs before a correct diagnosis is established. Thus, Steckelberg56 reported that the appearance of embolic events declined with time after therapy was started, from 13 per 1000 patient-days during the first week of therapy to fewer than 1.2 per 1000 patient-days after the second week of therapy.
Together with microbiological studies, TEE is the main tool for diagnosing endocarditis (Figure 5). Attempts have been made to identify echocardiographic factors that might identify a subgroup of patients at high risk for peripheral embolization. The location of the vegetation may define one risk group, although not all studies concur. Rohmann et al57 found that 25% of the patients with mitral involvement had peripheral embolism, versus only 9.7% of the patients with aortic endocarditis (P<.05). Likewise, the frequency of embolism increased when the vegetations involved the anterior mitral leaflet (37%). Others have reported similar results.58 However, contradictory results also exist, such as those by the groups of Mügge and Weyman, who failed to find any relation between the location of the vegetations and the risk of embolization.59,60
Fig. 5. Apical thrombus (T) in a left ventricle aneurysm after acute myocardial infarction. LA indicates left atrium; LV, left ventricle.
Vegetation size and mobility have received particular attention in studies related with the risk of embolization. However, varying results have been reported, probably due to the difficulty involved in standardizing the size of the vegetations and the use of different devices. General agreement exists, however, that vegetations larger than 10 mm are associated with a greater risk of thromboembolization. Heart surgery is indicated in patients with recurrent embolization, persistently positive cultures despite appropriate antibiotic therapy, heart failure and abscesses, and may be advised in patients with fungal endocarditis.
Atrial septal aneurysm
Atrial septal aneurysm (ASA) is a protrusion of the interatrial septum larger than 10 mm into either of the atrial chambers. Diagnosis is established at autopsy61 or by angiography.62 Clinically, TTE or TEE have now become the diagnostic techniques of choice (Figure 6) and it has been reported in up to 2.2% of all normal persons who undergo TEE.63 Importantly, however, the odds of ASA in young patients with stroke were up to four times as great as in an age-matched control group.63
Fig. 6. Subcostal echocardiography shows the presence of an atrial septal aneurysm protruding into the right atrium (RA). LA indicates left atrium.
Much controversy surrounds the reasons why ASA may cause peripheral emboli. It has been suggested that the aneurysm might be the site of formation of the thrombus. However, thrombi could only be detected within the aneurysm in 1% of a series of almost 200 patients with ASA.64 Nevertheless, in many patients ASA is associated with other sources of embolism such as patent foramen ovale (PFO), atrial fibrillation or mitral valve prolapse, any of which might be the true cause of the embolic event.
The recent publication of the results of the PFO-ASA study have clarified certain diagnostic aspects and have important clinical repercussions.65 A total of 581 patients with stroke of unknown origin were followed for four years. The risk of recurrent stroke was very low (2.3%) among the patients with PFO, moderate among the patients with both PFO and ASA (15.2%), and low among the patients with neither of these abnormalities (4.2%). No recurrences were seen among the patients with only ASA. Only the subgroup of patients with both PFO and ASA had a higher risk of recurrent stroke (4.2-fold increase). Aspirin is probably the treatment of choice in patients with either ASA or PFO. More aggressive therapy should be given to patients with both ASA and PFO diagnosed by TEE; such treatment might involve a combination of antiplatelet agents, oral anticoagulants or interventional closure of the PFO. Nevertheless, these alternative approaches should be evaluated in randomized trials.
Mitral valve prolapse
Although numerous studies have demonstrated the association between mitral valve prolapse and stroke, discrepancies exist as to the true value of this association. This is mainly because diagnostic criteria for mitral valve prolapse vary greatly, and because another cause of the thromboembolism may also be present in patients with mitral valve prolapse. Nishimura, in a longitudinal study of a large number of patients, found that 60% of those with stroke also had atrial fibrillation, thereby hindering determination of the direct cause of the mitral valve prolapse.66
Given the low absolute risk of stroke and the controversies arising in its diagnosis, which complicates the reporting of results, the recommendation to give primary prophylactic treatment to patients with isolated mitral valve prolapse does not seem justified.
Patients with mitral valve prolapse who develop symptoms of retinal or cerebral ischemia could be considered for treatment with antiplatelet agents. Results from the SPAF study suggest that warfarin might be recommended for patients with mitral valve prolapse and significant left atrial dilatation or atrial arrhythmia, or both.67
Patent foramen ovale
Evaluation of PFO is best done with TEE. Although Doppler color flow echocardiography reveals the presence of a shunt, peripheral intravenous injection via an arm vein and TEE is more sensitive for the detection of minimal shunts. The study is undertaken under basal conditions following the Valsalva maneuver, which increases right atrial pressure and disclosed any interatrial shunts not present under normal basal conditions (Figure 7).
Fig. 7. Transesophageal echocardiography in a patient with patent foramen ovale. After injection, contrast material can be seen passing into the left atrium. RA indicates right atrium; AO, aorta; LA, left atrium.
Transesophageal echocardiography has shown the incidence of PFO to be 3%-39%. Chen et al68,69 demonstrated that almost all patients with PFO undergoing surgery or catheterization were correctly identified with TEE. Detection of PFO is important because studies in patients with acute stroke and control subjects younger than 55 years of age showed PFO to be three times as common in patients with ischemic strokes as in the normal population.70 Furthermore, the combined annual rate of recurrent acute stroke in patients with PFO is 3%.
The reasons why PFO may be responsible for stroke in a patient with suspected cardiac embolism remain controversial. The possibility of paradoxical embolism via the PFO has been suggested. However, this attractive hypothesis remains questionable, as pre-existing deep vein thrombosis is usually understood to be the origin of the paradoxical embolism. An increasing number of reports confirm that the rate of deep vein thrombosis, as well as pulmonary embolism, is very low in patients with PFO and acute stroke, indicating that in many cases this may not be the causative mechanism.71 Other possibilities include direct embolism of a thrombus arising in the abnormal part of the septum,72 cerebral ischemia related with a greater incidence of paroxysmal atrial fibrillation in these patients,73 and the frequent association with ASA, which may be the true cause of the thromboembolism.74,75 The approaches to treatment are the same as for ASA.
Coagulation disorders
Of hypercoagulation states, the antiphospholipid syndrome is an example of a coagulation disorder associated with cardiac embolism and vascular thrombosis. Galve et al., one of the most active groups in the study this syndrome, reported mitral and aortic valve involvement in 38% of the patients with the primary antiphospholipid syndrome.76-79 The lesions are seen on echocardiography as localized thickening of the valve. Histologically, the lesion is composed of dense fibrous tissue with platelets and thrombi at the edges of the mass. Exceptionally, associated intracavitary thrombi with no other heart lesions are found (Figure 8).
Fig. 8. Transesophageal echocardiography in a patient with the primary antiphospholipid syndrome. There is slight thickening of the free edge of the anterior mitral leaflet. LA indicates left atrium; LV, left ventricle.
The finding of antiphospholipid antibodies in the absence of symptoms does not indicate that the patient should be treated. Patients with symptoms of thrombosis or stroke probably require treatment with warfarin. Some authors recommend keeping the INR between 3 and 3.5.80 In patiens with an isolated PFO, no other specific therapy shoold de initiated than, hypothetically, profilaxis against endocarditis.
Doppler echocardiography of supra-aortic trunks and transcranial Doppler
Diagnosis and monitoring of stroke
Transcranial Doppler (TCD) has now become a basic complementary diagnostic technique in patients with stroke because it is able to detect intracranial stenosis. Correlation studies comparing TCD with invasive methods involving arteriography have reported very high rates of sensitivity and specificity for the diagnosis of intracranial stenosis.81 Transcranial Doppler enables diagnosis of acute occlusion by transtemporal analysis of mean cerebral artery flow compared with the contralateral artery. Follow-up after the stroke shows when arterial recanalization occurs, and whether it is spontaneous or due to thrombolysis. Demchuk81 recently established a classification of the degree of reperfusion according to the flow characteristics on TCD, and confirmed that it predicts severity, early recovery and death in patients treated with fibrinolysis. However, the tortuous anatomy of the basilar artery may sometimes limit the usefulness of TCD.
Study of patent foramen ovale
A very important alternative technique to TEE for the evaluation of PFO is detection of microcavitations in the cerebral circulation after peripheral intravenous injection of agitated saline solution. The signals detected are generated by microbubbles which produce a short, high intensity Doppler signal on interaction with the ultrasound waves. This signal is known as a high intensity transient signal (HITS). Serena et al82 showed that it is possible to evaluate the severity of the shunt through the foramen ovale, and more importantly, defined a group of patients at high risk for stroke. The magnitude of the shunt is determined by counting the number of Doppler signals detected in the middle cerebral artery. Large shunts include those with more than 25 signals (shower pattern) or uncountable signals (curtain pattern) (Figure 8). The presence of either of these two patterns is associated with an increased risk of stroke (odds ratio, 3.5), particularly in patients with cryptogenic stroke (odds ratio, 12). This important study clearly defines the usefulness of TCD as a complement to TEE for the study of patients with patent foramen ovale, and the clinical implications of this anomaly.
Doppler echocardiography of supra-aortic trunks
Almost 30% of the patients with stroke have carotid disease. Thus far, coronary angiography has been the technique of choice to establish the severity of carotid disease and its therapeutic indications. The ECST83 and NASCET84 studies showed that surgical treatment is indicated for lesions larger than 75%-80%. Doppler echocardiography of the supra-aortic trunks shows not only the localization of the atheromatous plaque, but also its severity. The most commonly used parameter is maximum systolic velocity, which indicates more than 70% stenosis when flow velocity is greater than 215 cm/s. Almost half of all patients with TIA have carotid stenosis of less than 50%. In these patients it is necessary to evaluate the characteristics of the plaque and identify those patients whose alterations place them at high risk for arterial stroke.
Doppler monitoring of HITS has been described in patients with atrial fibrillation, artificial heart valves or intracranial stenosis, and during endarterectomy. Among patients with significant carotid disease HITS are more common in those with symptomatic carotid stenosis. They could, therefore, be an important marker of unstable plaque, thereby predicting the possibility of a clinical embolism.
DIAGNOSTIC PROCEDURES IN PATIENTS WITH ISCHEMIC STROKE
When a patient has symptoms of stroke, the first step is to determine whether it is ischemic or hemorrhagic in origin. This initial diagnosis conditions all subsequent decision-making and choice of therapy. Table 2 lists the tests which should be performed as soon as the patient reaches the hospital. Computed tomographic techniques are well standardized to distinguish between the two possible origins of the stroke, especially when we consider that the signs and symptoms between the two types largely overlap.
Once the type of stroke has been established, if the patient also has a suspected heart embolism there is no general agreement about when TTE or TEE should be performed. Our recommendations are given below (Table 3):
Indications for transthoracic echocardiography
Transthoracic echocardiography should be done in any patient with heart embolism, whether suspected or demonstrated by screening. By echocardiography and clinical examination we were usually able to diagnose from 8% to 10% of all patients; however, with the introduction of standard echocardiography this figure has risen to 15%-17%. This figure is still low, but it is nevertheless twice the figure when the technique is not used. Currently, echocardiography is a basic technique used systematically in clinical cardiology, and we do not believe that a low detection rate justifies its non-use. In our experience, almost 25% of the patients with proven carotid disease also have a secondary embolic cause. Therefore, a positive finding after supra-aortic echocardiography does not make TTE unnecessary.
Major indications for transesophageal echocardiography
The most commonly indication for this examination is undoubtedly stroke in a young person. In these cases the search for a PFO requires the use of TEE, which is also needed in any patient with suspected paradoxical embolism. Transesophageal echocardiography should also be performed in any patient with an artificial heart valve and symptoms of stroke. Finally, TEE should be done in patients with suspected heart embolism in whom the findings with TTE are uncertain, or who has a poor window.
Other indications for transesophageal echocardiography
Transesophageal echocardiography should probably also be done in patients with stroke and mitral valve lesions or emboligenic atrial fibrillation, as well as in patients with stroke in whom aortic arteriosclerosis is strongly suspected. These latter patients should be examined for the presence of complicating atheromatous plaques.
Fig. 9. Diagnosis of patent foramen ovale by Doppler study of the middle cerebral artery. Top: pattern suggesting more than a slight degree of shunting. Center: shower pattern. Bottom: curtain pattern.
Fig. 10. Clinical management based on image techiques in patients with sospeted stroke CT indicates computerized tomography; CSF, fluid;rt-PA, recombinant tissue plaominogen.
CONCLUSION
Cerebrovascular stroke, especially cardioembolic stroke, requires the combination of different hospital specialties for its correct evaluation. Specifically designed stroke units should be created for this purpose. Collaboration between the echocardiography laboratory and the neurology department is fundamental to develop and implement protocols for the correct diagnosis and care of patients with neurological problems. The notable advances over recent years have led to the discovery of new causes and an understanding of their importance as sources of heart embolism. Nevertheless, despite these advances, doubts still surround the choice of the best treatment options, and further research is required in this fascinating field of diagnosis.
Section sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. M.A. García Fernández.
Sección de Cardiología no Invasiva. Laboratorio de Ecocardiografía.
Hospital General Universitario Gregorio Marañón.
Dr. Esquerdo, 43. 28007 Madrid. España.
E-mail: Magfeco@primustel.es