Patients with advanced heart failure have a poor prognosis and heart transplant is still the best treatment option. However, the scarcity of donors, long waiting times, and an increasing number of unstable patients have favored the development of mechanical circulatory support. This review summarizes the indications for heart transplant, candidate evaluation, current immunosuppression strategies, the evaluation and treatment of rejection, infectious prophylaxis, and short and long-term outcomes. Regarding mechanical circulatory support, we distinguish between short- and long-term support and the distinct strategies that can be used: bridge to decision, recovery, candidacy, transplant, and destination therapy. We then discuss indications, risk assessment, management of complications, especially with long-term support, and outcomes. Finally, we discuss future challenges and how the widespread use of long-term support for patients with advanced heart failure will only be viable if their complications and costs are reduced.
Keywords
Heart failure (HF) is a clinical syndrome caused by reduced cardiac output and/or elevated intracardiac pressures.1 Its prevalence is 1% to 2% of adults in developed countries, constituting a major health problem with a high economic burden.2 Despite the availability of disease-modifying drugs and implantable device therapy, prognosis remains poor.
Approximately 5% of patients are in advanced HF as defined in Table 1.3 In a very small proportion of these patients, heart transplant (HT) is the only available treatment. Unfortunately, donors are limited, resulting in 250 to 300 HT per year in Spain and 2000 in the United States.4,5 Long waiting times1 and an increasing number of unstable patients have favored the development of mechanical circulatory support (MCS) as bridge to recovery, bridge to transplant (BTT), and bridge to candidacy or decision; initially with short-term ventricular assist devices (STVADs), and in the last decades with long-term ventricular assist devices (LTVADs). The development of LTVADs has created the possibility of destination therapy (DT) in patients who are not candidates for HT (Table 2).1
Definition of Advanced Chronic Heart Failure
| 1. Severe symptoms of heart failure with dyspnea and/or fatigue at rest or on minimal exertion (NYHA functional class III or IV) |
| 2. Episodes of fluid retention (pulmonary and/or systemic congestion, peripheral edema) and/or of reduced cardiac output at rest (peripheral hypoperfusion) |
| 3. Objective evidence of severe cardiac dysfunction, shown by at least 1 of the following: a) A low left ventricular ejection fraction (< 30%) b) A severe abnormality of cardiac function on Doppler echocardiography with a pseudonormal or restrictive mitral inflow pattern c) High left ventricular filling pressures (mean PCWP > 16mmHg, and/or mean RAP >12mmHg by pulmonary artery catheterization) d) High natriuretic peptide levels, in the absence of noncardiac causes |
| 4. Severe impairment of functional capacity shown by 1 of the following: a) Inability to exercise b) 6-minute walk test ≤ 300 m or less in females and/or patients aged ≥ 75 years c) Peak oxygen consumption < 12 to 14 mL/kg/min |
| 5. History of ≥ 1 heart failure hospitalization in the past 6 mo |
| 6. Presence of all the previous features despite “attempts to optimize” therapy including diuretics, renin-angiotensin-aldosterone system inhibitors, and beta-blockers, unless these are poorly tolerated or contraindicated, and cardiac resynchronization when indicated |
NYHA, New York Heart Association; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure.
Adapted from Metra et al.,3 with permission.
Indications for Mechanical Circulatory Support
| Bridge to decision/bridge to bridge | Use of short-term MCS in patients with cardiogenic shock until hemodynamic parameters and end-organ perfusion are stabilized, contraindications for long-term MCS are excluded (brain damage after resuscitation) and additional therapeutic options including long-term VAD therapy or heart transplant can be evaluated |
| Bridge to candidacy | Use of MCS (usually LVAD) to improve end-organ function, reverse pulmonary hypertension or overweight or provide enough time free of cancer, in order to make a patient eligible for heart transplant |
| Bridge to transplant | Use of MCS (LVAD or BiVAD) to keep a patient alive because of a high risk of death before transplant |
| Bridge to recovery | Use of MCS (LVAD or BIVAD) to keep a patient alive until cardiac function recovers sufficiently to remove MCS |
| Destination therapy | Long-term use of MCS (LVAD) in patients with end-stage heart failure ineligible for heart transplant |
BiVAD, biventricular assist device; LVAD, left ventricular assist device; MCS, mechanical circulatory support; VAD, ventricular assist device.
Adapted from Ponikowski et al.,1 with permission.
HT is the gold standard for the treatment of end-stage HF because it improves survival, functional status, and quality of life.6
IndicationsAlthough HT is the best option, it carries a mortality of approximately 15% in the first year.4,5 Therefore, assessing prognosis in patients with advanced HF is mandatory. The most often used scores are the Heart Failure Survival Score7 and the Seattle Heart Failure Model.8 A high- to medium-risk range in the first score or an estimated 1-year survival < 80% by the second are cutoff points for listing for HT. The BCNbioHF calculator provides prognostic information derived from clinical parameters but also incorporates biomarkers.9
Functional status evaluated with the cardiopulmonary exercise test is frequently used to determine HT eligibility. A peak oxygen consumption of < 14mL/kg/min or < 12mL/kg/min in patients on β-blockers at maximal exertion has been established as the cutoff point for HT.10 If exercise is submaximal, a ventilation to carbon dioxide slope of > 35 also has prognostic value. A 6-minute walk test < 300 meters also indicates high risk.
In patients with hemodynamic instability, HT may be performed emergently, preceded or not by MCS. To stratify patients in advanced HF, the Interagency Registry for Mechanical Assisted Circulatory Support (INTERMACS) created a classification that is prognostic and clinically useful regarding the need and type of MCS11 (Table 3). This classification has also been applied to patients transplanted in an emergency situation and showed worse prognosis for patients transplanted in INTERMACS 1-2 compared with INTERMACS 3-4.12 Therefore hemodynamic stabilization, either with medication or MCS, is strongly recommended prior to HT.
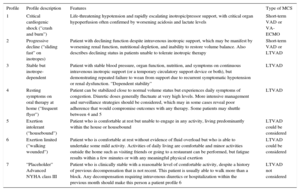
Interagency Registry for Mechanically Assisted Circulatory Support Profiles
| Profile | Profile description | Features | Type of MCS |
|---|---|---|---|
| 1 | Critical cardiogenic shock (“crash and burn”) | Life-threatening hypotension and rapidly escalating inotropic/pressor support, with critical organ hypoperfusion often confirmed by worsening acidosis and lactate levels | Short-term VAD or VA-ECMO |
| 2 | Progressive decline (“sliding fast” on inotropes) | Patient with declining function despite intravenous inotropic support, which may be manifest by worsening renal function, nutritional depletion, and inability to restore volume balance. Also describes declining status in patients unable to tolerate inotropic therapy | Short-term VAD or LTVAD |
| 3 | Stable but inotrope-dependent | Patient with stable blood pressure, organ function, nutrition, and symptoms on continuous intravenous inotropic support (or a temporary circulatory support device or both), but demonstrating repeated failure to wean from support due to recurrent symptomatic hypotension or renal dysfunction. “Dependent stability” | LTVAD |
| 4 | Resting symptoms on oral therapy at home (“frequent flyer”) | Patient can be stabilized close to normal volume status but experiences daily symptoms of congestion. Diuretic doses generally fluctuate at very high levels. More intensive management and surveillance strategies should be considered, which may in some cases reveal poor adherence that would compromise outcomes with any therapy. Some patients may shuttle between 4 and 5 | LTVAD |
| 5 | Exertion intolerant (“housebound”) | Patient who is comfortable at rest but unable to engage in any activity, living predominantly within the house or housebound | LTVAD could be considered |
| 6 | Exertion limited (“walking wounded”) | Patient who is comfortable at rest without evidence of fluid overload but who is able to undertake some mild activity. Activities of daily living are comfortable and minor activities outside the home such as visiting friends or going to a restaurant can be performed, but fatigue results within a few minutes or with any meaningful physical exertion | LTVAD could be considered |
| 7 | “Placeholder” Advanced NYHA class III | Patient who is clinically stable with a reasonable level of comfortable activity, despite a history of previous decompensation that is not recent. This patient is usually able to walk more than a block. Any decompensation requiring intravenous diuretics or hospitalization within the previous month should make this person a patient profile 6 | LTVAD not considered |
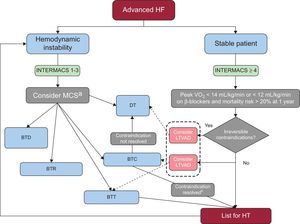
A decision-making algorithm for HT is provided in Figure 1.
Decision-making algorithm for patients with advanced HF as defined in Table 1 after appropriate optimization of medical, device, and surgical treatment. BTC, bridge to candidacy; BTD, bridge to decision; BTR, bridge to recovery; BTT, bridge to trasplant; DT, destination therapy; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; HF, heart failure; HT, heart transplant; LTVAD, long-term ventricular assist device; MCS, mechanical circulatory support; VO2, oxygen consumption. aIn patients in INTERMACS 1 a short-term ventricular assist device should be placed, preferably venoarterial extracorporeal membrane oxygenation in conditions such as unclear neurological status, unstable hemodynamics and severe coagulopathy. In less catastrophic situations and in INTERMACS 2, a uni- or biventricular short-term ventricular assist device such as the Centrimag can be implanted, as it can provide up to 1 month of support. After resuscitation of the patient, a weaning trial of the device must be performed and, if not possible, assessment for HT is crucial. The next step should be exchange to a LTVAD as BTT or in some cases as DT. In patients in INTERMACS 3, a LTVAD, preferably only supporting the left ventricle, is recommended. bAfter bridge to candidacy, if the contraindication (pulmonary hypertension, time free of cancer or excess weight) is resolved, the patient should be listed.
A holistic approach is imperative for selecting the best recipients for a scarce resource. The expected mortality after HT can be calculated with the IMPACT model.13 Comorbidities that increase the morbidity and mortality of HT can amount to absolute or relative contraindications and are described in Table 4.
Contraindications for Heart Transplant
| Absolute |
| Systemic disease with life expectancy < 2 years: Active neoplasm (if preexisting, evaluation with an oncology specialist is necessary to stratify the risk of recurrence and establish a time to wait after remission) Systemic disease with multiorgan involvement (systemic lupus erythematosus, amyloidosis, sarcoidosis) Severe chronic obstructive pulmonary disease (FEV1 < 1 L) Renal or hepatic severe dysfunction, if associated renal or liver transplant is not performed Irreversible pulmonary hypertension Pulmonary artery systolic pressure > 50mmHg Transpulmonary gradient > 12mmHg Pulmonary vascular resistance > 3 Wood units despite treatment |
| Relative |
| Age > 70 years (carefully selected patients may be considered) Diabetes with end-organ damage (except nonproliferative retinopathy) or persistent poor glycemic control (HbA1c > 7.5%) despite treatment Active infection, except VAD infection. Patients with HIV, hepatitis, Chagas disease and tuberculosis can be considered with strict management Severe peripheral arterial or cerebrovascular disease not suitable for treatment Other serious comorbidity with poor prognosis, such as neuromuscular diseases Obesity: BMI > 35 kg/m2 Cachexia: BMI < 18 kg/m2 Frailty: when 3 of 5 possible symptoms (including unintentional weight loss of > 5kg within the past year, muscle loss, fatigue, slow walking speed, and low levels of physical activity) are present Current alcohol or drug abuse Insufficient social support Elevated panel-reactive antibody test defined as > 10% |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; HbA1c, glycated hemoglobin; HIV, human immunodeficiency virus; VAD, ventricular assist device.
Pulmonary hypertension develops in response to a passive backward transmission of elevated filling pressures in the left ventricle that with time causes vascular remodelling. Right heart catheterization is recommended before HT, as irreversible pulmonary hypertension is associated with right HF and higher mortality.14
Reactive pulmonary hypertension is defined as the presence of a transpulmonary gradient > 12mmHg and a pulmonary vascular resistance > 3 Wood Units. Pulmonary vasoreactivity testing with inotropes, vasodilators, and diuretics is necessary to demonstrate reversibility, defined as a reduction of transpulmonary gradient to < 12mmHg and pulmonary vascular resistance < 3 Wood Units. Unless these values are achieved, HT is contraindicated.10 In patients listed for HT, the reversibility of pulmonary hypertension should be reassessed at 3- to 6-month intervals.
Bosentan and sildenafil can sometimes reverse pulmonary hypertension in 3 to 4 months and make the patient suitable for HT.15 However, guidelines do not support their use in patients with left HF.14 Left ventricular assist devices (LVADs) can also lower pulmonary hypertension and are used as a bridge to candidacy in patients with irreversible pulmonary hypertension refractory to medical treatment.16
Current PictureIn the last few years, a trend has been observed toward older recipients with complex clinical profiles, suboptimal donors (54%) and relatively long ischemia times.4,5 There is a worrying tendency toward an increase in emergency transplants (the criteria in Spain can be seen in Table 5) representing up to 40% (20% with MCS) of transplants in Spain4; in 2013, 50% of candidates were bridged with MCS according to the international registry.5 These figures reflect an era of donor shortage and an increased acceptance of more complex candidates for HT. Despite this situation, early mortality after HT remains similar to that of previous periods.4,5
Emergent Transplant Criteria in Spain
| Grade 0 emergency (national priority) | Advanced HF with short-term MCS (including ECMO) Dysfunctional long-term VAD - Mechanical dysfunction - Infection - Tromboembolism |
| Grade 1 emergency (regional priority) | Advanced HF with 1 of the following: - Vasoactive drugs and invasive mechanical ventilation - Intra-aortic balloon pump - Long-term VAD Arrythmogenic storm defined as 3 or more sustained ventricular tachycardia, polymorphic ventricular tachycardia or ventricular fibrillations in 24 h that require intervention to finish them despite maximal antiarrythmic treatment |
ECMO, extracorporeal membrane oxygenation; HF, heart failure; MCS, mechanically circulatory support; VAD, ventricular assist device.
Information regarding surgery, organ preservation, and donor selection can be found elsewhere.17
ImmunosuppressionLong-term outcome depends on the maintenance of the minimum immunosuppression levels necessary to avoid rejection and minimize adverse effects.
Induction TherapyInduction therapy guarantees rapid and profound immunosuppression immediately after HT. Thymoglobulin has traditionally been used and is highly effective in depleting lymphocytes. The lack of randomized trials and increased infection rate has currently limited its use to sensitized patients and those at high risk of acute rejection. Dosing thymoglobulin according to lymphocyte count reduces the cumulative dose without compromising efficacy.18
Currently, in patients with a lower rejection risk, induction therapy is performed with interleukin-2 receptor antagonists, such as basiliximab, which has a better safety profile.19 Differences in the use of induction therapy per protocol exist between centers and, while induction therapy consists of basiliximab in 85% of patients in Spain, it is only used in 30% of patients in the international cohort.4,5
Guidelines recommend induction therapy in patients at high risk for acute rejection or renal dysfunction with the aim of delaying the use of calcineurin inhibitors.17–19 Although widely employed, their impact on survival and long-term adverse effects are unknown.
Immunosuppression Maintenance StrategiesImmunosuppression must be higher in the first 3 to 6 months. Over the years, it can be reduced according to individual risk and the results of endomyocardial biopsy. Initially, standard therapy includes the synergistic combination of 3 groups of drugs.17
- •
Calcineurin inhibitors: cyclosporine or tacrolimus. Both are metabolized by CYP-450 and consequently have many drug interactions. Adverse effects (arterial hypertension, diabetes, dyslipidemia, neurotoxicity) depend on blood levels, and therefore close monitoring is mandatory. Regarding efficacy, tacrolimus may be associated with lower rejection rates without differences in infection or survival.20 Arterial hypertension and dyslipidemia are less frequent with tacrolimus, whereas diabetes risk is increased. Currently, 85% of patients receive tacrolimus during the first year.4,5
- •
Antiproliferative drugs inhibit de novo synthesis of purines and block cytotoxic and B lymphocyte proliferation. Added to calcineurin inhibitors, they allow a reduction of their levels. Mycophenolate mofetil is chosen in 96% of patients3 since it exhibits better survival, less rejection, and less cardiac allograft vasculopathy than azathioprine.21 The most frequent adverse effects are leukopenia and gastrointestinal intolerance (the delayed-release formulation is better tolerated in this regard).
- •
Corticosteroids. A wide range of adverse effects (arterial hypertension, diabetes, hyperlipidemia, osteopenia, myopathy, emotional instability, infection) requires their reduction and, if possible, their discontinuation after 6 months. Withdrawal must be closely monitored by biopsy, as a high rate of acute rejection has been observed. Currently, 80% of patients remain on corticosteroids after 1 year4 and 62% at 7 years.5
Alternatives to the first 2 drugs are mammalian target of rapamycin (m-TOR) inhibitors (everolimus, sirolimus), which inhibit the proliferation of lymphocytes and smooth muscle cells. Everolimus combined with calcineurin inhibitors has been shown to reduce rejection and cardiac vasculopathy compared with mycophenolate mofetil, without differences in survival.22 The SCHEDULE trial demonstrated a reduction in cardiac vasculopathy progression in the group with early everolimus introduction and calcineurin inhibitor withdrawal compared with standard calcineurin inhibitor therapy, but a higher rejection rate was also seen.23 While a combination of an m-TOR inhibitor and calcineurin inhibitor is recommended in cardiac vasculopathy, a strategy of calcineurin inhibitor withdrawal or minimization is recommended in renal dysfunction and cancer.24,25 Even though m-TOR inhibitors have been shown to be useful, a significant decrease in their use has been noticed in the last few years4,5 mainly driven by their numerous adverse effects: infections, pneumonitis, proteinuria, effusions, hyperlipidemia, diarrhea, and myelotoxicity.26
Single use of tacrolimus in the long-term has also been proposed, without differences in rejection in the first year.27 However, this is not a common approach (10%)5 and further studies are necessary.
RejectionGraft rejection is one of the main causes of death in the first few years after HT. Hyperacute rejection due to preformed antibodies against AB0 or human leucocyte antigens is rare at present.
Cellular Acute RejectionCellullar acute rejection is mediated by T-lymphocytes and is characterized by inflammatory infiltration with myocyte damage.17 Its severity is classified according to the pathology findings.10 Only symptomatic patients or asymptomatic patients with severity ≥ 2R (multifocal myocyte damage) are treated with corticosteroids +/- thymoglobulin if there is hemodynamic instability. The incidence of treated rejection in the first year is around 15%.5
Surveillance biopsies for detecting rejection are routinely performed in most centers, more frequently in the first 3 months; they are then tapered until 1 year post-transplant and afterwards only if rejection is clinically suspected.17,19
Allomap is a noninvasive gene-expression profiling test for rejection surveillance in HT recipients. This test has a high negative predictive value, identifies patients at low risk for cellular rejection, and can avoid routine biopsies a few months after HT.28
Antibody-mediated RejectionThis is a recently described entity, present in 10% to 20% of HT. B-lymphocytes produce antibodies against human leucocyte antigens activating an inflammatory response that causes endothelial dysfunction. Prevention by minimizing exposure to alloantigens (avoiding nonessential blood transfusions) and maintaining appropriate immunosuppression is paramount. Diagnosis is challenging and suspicion is the key. The usual clinical picture is a patient with HF, left ventricular dysfunction without cellular infiltration in the biopsy, female sex, allosensitized, previous transfusion, retransplanted, and prior LVAD or history of Cytomegalovirus infection. Confirmation is made by pathology findings (vasculitis, edema), C4d or C3d deposition, and determination of antihuman leucocyte antigen antibodies in serum. Therapy and its duration are not well established but there is a consensus in treating patients with biopsy findings and dysfunction or antibodies. A more detailed review of diagnosis and treatment can be found elsewhere.29
Cardiac Allograft VasculopathyThis entity is characterized by diffuse and concentric thickening of the intima of the epicardial and intramural coronary arteries. Its etiology is not clear but it is considered to be the manifestation of chronic rejection influenced by nonimmunological factors, such as diabetes mellitus, hypertension, smoking, and Cytomegalovirus infection. Its clinical expression includes angina, myocardial infarction, or sudden death but it usually manifests as HF with or without ventricular dysfunction. Statin therapy has been shown to reduce graft vasculopathy and mortality and therefore it is indicated in all HT patients irrespective of lipid levels.17 A baseline coronary angiography 1 month after HT and another at 1 year are recommended. After that, the need for repeat coronary angiographies or noninvasive tests is variable.17,19
Infectious Complications: ProphylaxisDiagnosis of infection can be challenging in HT recipients and treatment must be aggressive. Prevalence is higher in the initial 6 months. In the very early period, previous recipient infections can be exacerbated and donor-transmitted or surgery-related infections may appear. Between 0.5 to 6 months, opportunistic infections emerge: viral (Cytomegalovirus), fungal (Aspergillus and P. jiroveci) and bacterial (Nocardia and Lysteria). After 6 months, the risk diminishes and community-acquired infections are the most common.
Prophylaxis should be started 10 to 15 days post-HT: ganciclovir or valganciclovir for Cytomegalovirus for 3 months or preemptive therapy guided by polymerase chain reaction determinations. In the case of Cytomegalovirus donor positive/recipient negative recipients, prophylaxis for 6-12 months is recommended. Trimethoprim/sulfamethoxazole is recommended for P. jirovenci and Toxoplasma gondii for 6 months in all patients, or for up to 1 year if treated with everolimus; antifungal prophylaxis with nystatin to prevent candidiasis while the patient is on high dose corticosteroids and inhaled amphotericin B for Aspergillus during the initial hospitalization. Further information about dosage and regimens can be found in various guidelines.17–19
Causes of Death After Heart TransplantOverall, survival at 1, 5, and 10 years is 81%, 68%, and 51%4. In the Spanish cohort, median survival is 10.9 years. Survival is worse in older recipients, with older donors, emergency transplant, and recipients supported by venoarterial extracorporeal membrane oxygenation (VA-ECMO).4,5
Primary graft failure is the leading cause of death in the first month, while infection is the most common cause during the first year. Primary graft failure is defined as ventricular dysfunction without a clear etiology. The RADIAL score can help stratify risk.30 After 1 year, the main causes of death are cardiac vasculopathy and malignancy.4 Globally, the main cause of death in Spain is cardiac vasculopathy (20%), followed by infection (16%), primary graft failure (14%) and tumours (13%).4
MECHANICAL CIRCULATORY SUPPORTThe use of MCS has grown exponentially over the past 15 years, mostly as BTT. However, other strategies after implantation of MCS exist, as noted previously.
Type of Mechanical Circulatory SupportThe type of MCS will depend on the clinical situation, defined by the INTERMACS classification.
Two groups are distinguished:
- •
Short-term ventricular assist devices aim to support hemodynamically unstable HF patients for days or weeks. Initially used for postcardiotomy shock, their use was expanded to stabilize a shocked patient and gain time either for recovery, decision, or BTT.
- •
Long-term ventricular assist devices are designed to assist patients with advanced HF during a period of months to years, while awaiting candidacy, transplant and, in very few cases, recovery. However, with the appearance of continuous-flow LTVADs and their increased durability, DT has become an option. Currently, many patients who receive a LTVAD are DT equivalent, because only 30% of patients with MCS implanted as BTT will receive an organ within the first year of listing.31
Additionally, VADs can be classified according to various criteria: a) ventricle supported: left, right or both (biventricular VAD or total artificial heart); b) location: extracorporeal or intracorporeal; c) flow provided: pulsatile-flow or continuous-flow, and d) pump: pneumatic, axial, or centrifugal.
Short-term Ventricular Assist DevicesPercutaneous STVAD will not be discussed, as these devices are reviewed in another article published in Revista Española de Cardiología.
In our environment, VA-ECMO and Centrimag (St. Jude Medical, Pleasanton, California, USA) are the preferred options in INTERMACS 1-2. Venoarterial ECMO is a modified cardiopulmonary bypass that supports both ventricles (3.5-4.5 L/min), with the possibility of peripheral cannulation, even outside of the operating room. The Centrimag is a central continuous-flow pump (4-7 L/min) that can be used as left, right, or biventricular support via sternotomy. When to use one or the other is discussed in Figure 1.
Despite the use of MCS in cardiogenic shock, mortality is still around 50%, mainly due to shock prior to implant and less frequently secondary to complications of the STVAD.32 What is important is to design a strategy after implanting a STVAD, aiming for recovery when possible, but it is also important to have the possibility of bridging from VA-ECMO to Centrimag, from STVAD to HT if waiting times are acceptable, or from STVAD to LTVAD. Implantation of a STVAD in a community hospital and then bridging to a LTVAD in a tertiary hospital can be performed with similar results to those achieved when the whole process is performed in a tertiary hospital,33 emphasizing the importance of early stabilization with STVADs in cardiogenic shock.
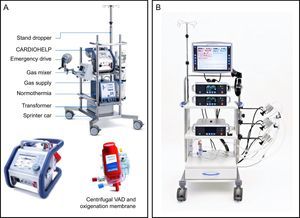
In Spain, 47% of patients undergo HT in an emergency situation: > 50% of them with an intra-aortic balloon pump, 23% with ECMO, 16% with continuous-flow VADs, and 6.5% with pulsatile-flow VADs.4 Venoarterial ECMO is clearly associated with worse survival after HT4,5 compared with the remaining options. However, the registry does not distinguish between STVADs and LTVADs and therefore outcomes after HT in patients with a STVAD, such as the Centrimag, are unknown.34 This issue will be clarified by an analysis of STVAD as BTT that is currently underway (ASIST-TC study). The most common STVADs are shown in Figure 2.
Short-term ventricular assist devices. A: CARDIOHELP (Maquet, Bridgewater, New Jersey, United States) extracorporeal membrane oxygenation system. B: Centrimag (St. Jude Medical, Pleasanton, California, United States) is a continuous-flow centrifugal pump that can support one or both ventricles. VAD, ventricular assist device. Reproduced with the permission of Maquet and St. Jude Medical.
In the seventh annual report of the INTERMACS database, more than 15 000 LTVADs have been reported with a mean rate of 2500 patients per year in the last 2 years.31 Although initially conceived for as BTT, their usefulness as DT was first evaluated in the REMATCH trial.35 This trial used pulsatile-flow LTVADs and, although they provided adequate support, their use was limited by their short durability, cost, and large size. The introduction of continuous-flow LTVADs led to better survival free from stroke and device failure.36 Currently > 90% of LTVADs are continuous-flow.31 This improvement in technology has also led to an increase in DT to 46% of all implants in the United States.31
In Spain, the most commonly employed LTVAD until recent years was the Excor (Berlin Heart, Berlin, Germany). However, because of the need for high levels of anticoagulation and antiplatelet therapy to avoid thromboembolic complications,37 most centers currently use it only for mid-term support or as biventricular support. Currently, continuous-flow LTVAD are the preferred option for long-term support.
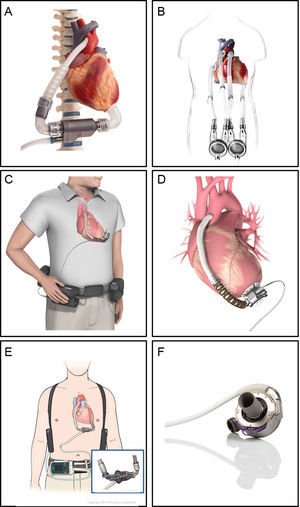
The VADs currently being used are shown in Figure 3. The INCOR was the first LTVAD used in Spain. The HeartMate II is the most frequently used continuous-flow LTVAD, and the only one approved for DT. The others are smaller centrifugal pumps, avoiding the need for an abdominal pocket. In the ADVANCE and ENDURANCE trials, the HVAD was shown to be noninferior to the HeartMate II. Initial experience with HeartMate III has shown better outcomes at 6 months compared with HeartMate II, primarily because of a lower rate of pump thrombosis.38
Long-term ventricular assist devices. A: INCOR (Berlin Heart, Berlin, Germany) is an intracorporeal continuous-flow axial pump with a magnetically levitated impeller. B: EXCOR (Berlin Heart, Berlin, Germany) is a paracorporeal pulsatile-flow pump with 1 or 2 ventricles. C and D: HVAD (HeartWare, Framingham, Massachusetts, United States) is an intracorporeal continuous-flow centrifugal pump. E: HeartMate II (St. Jude Medical, Pleasanton, California, United States) is an intracorporeal continuous-flow axial pump. F: HeartMate 3 (St. Jude Medical, Pleasanton, California, United States) is an intracorporeal continuous-flow magnetically levitated centrifugal pump. Reproduced with the permission of Berlin Heart, St. Jude Medical and HeartWare.
Heart failure patients eligible for LVAD implantation must fulfil the criteria described in Table 6.1
Patients Potentially Eligible for Implantation of a Left Ventricular Assist Device
| Patients with > 2 mo of severe symptoms despite optimal medical and device therapy and more than 1 of the following: |
| Left ventricular ejection fraction < 25%, and if measured, peak VO2 < 12 mL/kg/min ≥ 3 hospitalizations for HF in the previous 12 mo without an obvious precipitating cause Dependence on intravenous inotropic therapy Progressive end-organ dysfunction (worsening renal and/or liver function) due to reduced perfusion and not to inadequate ventricular filling pressure (PCWP ≥ 20mmHg and SBP ≤ 80-90mmHg or CI ≤ 2 L/min/m) Absence of severe right ventricular dysfunction together with severe tricuspid regurgitation |
CI, cardiac index; HF, heart failure; PCWP, pulmonary capillary wedge pressure; SBP, systolic blood pressure; VO2: oxygen consumption.
Adapted from Ponikowski et al.,1 with permission.
A thorough evaluation of risk is mandatory39 and overall, outcomes of patients with continuous-flow-LVAD are satisfactory. The current survival rates are approximately 80% and 70% at 1 and 2 years as BTT and 75% and 65% as DT,31 but are worse with biventricular support (50% at 1 year). Transplant outcomes in patients bridged with continuous-flow LTVAD are similar to those without bridging.40
For patients in INTERMACS 1,31 LTVAD implantation should be avoided since their survival is lower. Short-term ventricular assist device should be considered in these patients instead.
Regarding ambulatory HF, the ROADMAP41 trial studied patients in INTERMACS ≥ 4. The 1-year freedom from death, urgent HT, or delayed LTVAD was better for the LTVAD group compared with medical treatment (80% vs 63%, P = .024). However, adverse events were twice as common with LTVADs, indicating the need for caution regarding too early LTVAD placement.
The HeartMate II risk score identified hypoalbuminemia, renal dysfunction, coagulopathy, center experience, and age as risk factors for 90-day mortality.42 Although the studies performed have not shown differences in the incidence of complications in patients > 70 years,43 these patients have worse survival.31 In most centers, LTVADs are used in patients up 75 years of age and after that a “case by case” evaluation is made. Furthermore, malnutrition, frailty, and sarcopenia also predict worse outcomes, and preoperative optimization and psychosocial assessment are essential.
In general, LTVADs have been demonstrated to improve quality of life and functional status.44
Right Ventricular Evaluation and Preimplant OptimizationRight ventricular failure is a leading cause of morbidity, mortality, and increased length of stay after LVAD implantation.
Predictive models can help evaluate risk. Clinical and laboratory parameters predictive of poor outcomes include female sex, previous surgery, nonischemic cardiomyopathy, vasopressor support, bilirubin ≥ 2.1mg/dL, aspartate aminotransferase ≥ 80 IU, albumin < 3.4mg/dL, and creatinine ≥ 1.9mg/dL. Elevated central venous pressure and central venous pressure/wedge ratio 0.63 are the best hemodynamic predictors of right ventricular failure. Cardiac index < 2.2 L/min/m2, systolic blood pressure < 96mmHg, and right ventricular stroke work index < 330g/m2/beat also predict right ventricular failure. The best echocardiographic predictors of right ventricular failure are: right ventricle/left ventricle ratio > 0.72, short-to-long axis diameter ratio of the right ventricle >0.6, tricuspid annulus peak systolic velocity <8cm/s, systolic peak longitudinal strain <0.6cm/s, and severe tricuspid regurgitation with systolic pulmonary pressure < 50mmHg.45
Preoperative optimization to lower central venous pressure < 15mmHg prevents right ventricular failure. Inhaled nitric oxide or sildenafil can be used to decrease pulmonary pressure, but whether they decrease right ventricular failure is uncertain.46 Milrinone or levosimendan may also be useful, and sometimes intra-aortic balloon pump may be necessary prior to LTVAD implantation.
A tricuspid annulus > 40mm and moderate-severe tricuspid regurgitation have been proposed as indicators for tricuspid valve repair. However, this procedure failed to reduce early mortality or need for right ventricular support in LVAD patients and it associated more prolonged length of stay.47 Low pump speeds are initially recommended and further adjustments may be necessary according to echo and hemodynamic parameters.
Despite adequate stratification and medical management, right ventricular failure may occur (0.49 events/100 patients-month).31 Patients who need right VAD support have worse outcomes. However, if the implant occurs during the LVAD surgery, survival is better.48 Late right ventricular failure may appear in up to 11% of patients and is associated with worse survival in BTT.49
Complications and Management of Left Ventricular Assist Device PatientsInitially, the standard treatment to prevent thromboembolic complications is aspirin 81 to 325mg once daily to achieve an araquidonic acid inhibition > 70%, and vitamin K antagonists to achieve an international normalized ratio of 2 to 3. Clinical practice has established a maximum goal of an international normalized ratio of 2.5 in the absence of other thromboembolic risk factors, as bleeding occurs more frequently than thrombosis, although antithrombotic therapy must be tailored to the specific device and patient. Bridging with heparin in the initial postoperative period is generally recommended.
One of the most feared complications is stroke, which can occur in 7% to 15% of patients with an LVAD. Predictors of ischemic stroke with the HVAD are aspirin ≤ 81mg and atrial fibrillation, whereas predictors of hemorrhagic stroke are a mean arterial pressure > 90mmHg, aspirin ≤ 81mg, and an international normalized ratio > 3. To decrease the incidence of hemorrhagic strokes, it is crucial to strictly control blood pressure so that the mean arterial pressure is < 90mmHg.50
Bleeding events are favored by shear stress on blood components and reduced pulse pressure in continuous-flow technology. The current incidence (7.79 events/100 patient-months) is lower than in previous periods.31 The most prevalent is gastrointestinal bleeding, which is a leading cause of rehospitalization but does not affect survival. Described predisposing factors are age, lower albumin levels, and lower body mass index.51 Pathophysiology is explained by acquired von Willebrand factor deficiency, impaired platelet aggregation, and gastrointestinal angiodysplasias due to reduced pulse pressure akin to Heyde syndrome.52,53
The TRACE US study54 analyzed 100 patients with reduced antithrombotic therapy after a bleeding episode. Despite this, subsequent bleeding occurred in 52%, although rates of ischemic stroke were similar.
The incidence of pump thrombosis rose from 2.2% at 3 months after implantation in 2011 to 8.4% in 2013,55 probably due to changes in clinical management with lower goals of anticoagulation and antiaggregation and lower LVAD flows in order to achieve opening of the aortic valve. Pump thrombosis is a daunting complication that depends on device characteristics, operative technique, antithrombotic management, and patient factors. This complication must be suspected in the presence of hemolysis (lactate dehydrogenase elevation and/or high plasma free hemoglobin), a transient pump power increase, or left HF. The best diagnostic tool for Heartmate II is the Columbia ramp study,56 in which blunted reductions in left ventricular end diastolic diameter in response to increases in pump speed indicate an obstruction to flow through the device. These slope parameters cannot be directly applied to HVAD patients, as the left ventricle end diastolic diameter slope is drastically smaller.57 Treatment is based on increasing antithrombotic medication and if there is hemodynamic instability, fibrinolysis or pump exchange may be performed.
Another frequent complication is aortic insufficiency, which is noted in 25% to 52% of patients after 1 year of continuous-flow-LVAD support and is cumulative over time. The reasons for the development of aortic insufficiency are thought to be: a) lack of opening of the aortic valve, which may lead to leaflet fusion, and b) altered flow dynamics in the ascending aorta, which may contribute to aortic sinus dilatation. Therefore, when more than mild aortic insufficiency is detected prior to LVAD implantation, it is recommended to repair or replace the aortic valve. To prevent aortic insufficiency after LVAD implantation, it is recommended to optimize speed to eliminate more than mild MR and position the septum at the midline. If both are achieved, speed may be reduced to allow intermittent aortic valve opening. If aortic insufficiency secondary to LVAD is asymptomatic, speed reduction to maximize aortic valve opening is recommended. If the patient is symptomatic, an increase in speed is recommended. If symptoms persist after hemodynamic assessment, repair, replacement or closure of the aortic valve with a patch or by sewing the leaflets may be considered.58
Driveline infection is a feared complication present in up to 40% of patients over time. The incidence of infection can be decreased by patient education and the use of a standardized kit with a silver dressing and an anchoring device.59
Finally, the development of human-leucocyte antigen antibodies during MCS has been described. Younger age, pre-VAD panel-reactive antibodies, and female sex were independent predictors of elevated antibodies post-VAD. Although the development of these antibodies is associated with a longer waiting time for HT due to the need for virtual cross-match, no association with increased rejection, graft failure or death after HT was found.60
FUTURE PROSPECTSThe incipient use of donor hearts after circulatory death may increase the donor pool.61 However, this increase might not be sufficient to meet the needs of all the patients with advanced HF. To increase the use of LTVADs in the future, we would need to reduce their cost, diminish thrombotic and bleeding complications and avoid the driveline to minimize infections.
In Spain, the use of LTVADs as BTT or bridge to candidacy is slowly increasing, but their use as DT is still anecdotal and is restricted to young patients with an absolute contraindication for HT and those aged 65 to 75 years with comorbidities that may limit graft survival. The main reason for the low rates of LVAD use is the high cost of the device, which is approximately 94 600€. With a current threshold of 30 000€/quality-adjusted life years62 for end-of-life care interventions, by reducing the cost by 15%, the incremental cost-effectiveness ratio may be acceptable.
CONCLUSIONSHT remains the best available therapy for patients with advanced HF but, given the shortage of donors and long waiting lists, LVADs are increasingly being used to save lives and enhance quality of life. In Spain, a shift from STVADs to LTVADs as BTT or bridge to candidacy is occurring but the pace is slow. Implantation of LTVADs as DT can be an alternative for highly selected patients, but access to these devices is limited by their cost. Widespread use of LTVADs will only be viable if their complications are reduced and become cost-effective.
CONFLICTS OF INTERESTU.P. Jorde is a consultant to St. Jude Medical (no honoraria).