To compare acute myocardial infarction patients with or without congestive heart failure in the French FAST-MI registry.
MethodsThe French FAST-MI registry included 374 centers and 3059 patients over a 1-month period at the end of 2005, with 1-year follow-up. Among this population, patients with at least one congestive heart failure criterion constituted group 1 (n=1149; 37.5%) and were compared to patients without congestive heart failure (group 2, n=1910; 62.5%). The congestive heart failure patients were further divided according to presence of both beta-blockers and antagonists of the renin-angiotensin-aldosterone system at hospital discharge (n=511) or not (n=498), in order to assess the real-world clinical importance of recommended medications.
ResultsOverall in-hospital and 1-year mortality rates were 3.4% and 13.2%, respectively. In hospital survivors, presence of congestive heart failure was associated with increased mortality (adjusted hazard ratio=1.55; 95% confidence interval, 1.10-2.17; P=.01). Survival was higher in patients without congestive heart failure, compared with congestive heart failure patients receiving or not recommended medications (P<.001). Congestive heart failure patients receiving neither renin-angiotensin-aldosterone system blockers nor beta-blockers (adjusted hazard ratio=1.66; 95% confidence interval, 1.08-2.55; P=.02) had a significantly higher risk of death than patients receiving both classes of medications (adjusted hazard ratio=1.16; 95% confidence interval, 0.82-1.64; not statistically significant). Patients receiving only one of the recommended classes had an intermediate risk (adjusted hazard ratio=1.47; 95% confidence interval, 1.04-2.07; P=.03).
ConclusionsPatients admitted for acute myocardial infarction with congestive heart failure criteria are still at very high risk of mortality. When receiving major recommended medications, they presented with significantly reduced mortality rates. Additional efforts should therefore be made to encourage the prescription of recommended medications in acute myocardial infarction patients with congestive heart failure.
Trial registration: ClinicalTrials.gov number: NCT00673036.
Keywords
.
IntroductionClinical trials have shown that combining beta-blockers (BB) and angiotensin-converting enzyme (ACE) inhibitors has an addictive effect in reducing short- and long-term mortality and morbidity in patients with congestive heart failure (CHF) due to either left ventricular systolic dysfunction1, 2, 3 or acute myocardial infarction (MI) with left ventricular dysfunction with or without symptoms of CHF.4, 5, 6, 7 Guidelines have been published, specifying the optimal treatment strategies for such patients.8 For each type of CHF conditions, several important registries have provided useful information on the prescription of recommended drugs and their impact on long-term mortality, showing an important under-prescription despite a major role on reducing mortality.9, 10 Available data from registries have shown the importance of recommended drugs used in the context of either MI9, 11, 12 or CHF.10 However, the real impact of these drugs on survival when combining CHF in acute MI in the context of real life has not been demonstrated. The French nationwide Acute ST-elevation and non-ST-elevation Myocardial Infarction (FAST-MI) 2005 survey is a prospective registry of all patients admitted to an intensive care unit (ICU) in France for acute MI by the end of 2005.13 It gives the opportunity to analyze the specific characteristics of patients presenting with CHF criteria within the population of patients with acute MI.
The purpose of the present study is to compare the baseline clinical profile, drug prescriptions at hospital discharge, and short- and long-term mortality and morbidity in acute MI patients with or without CHF.
Methods PopulationThe objectives of the FAST-MI 2005 registry were to gather complete and representative data on the management and outcome of patients admitted to ICU for acute MI during a 1-month period in France, irrespective of the type of institution to which the patients were admitted (that is, university hospitals, public hospitals, or private clinics). The FAST-MI design has been already described in detail.13 In summary, from a list of all ICUs or coronary care units admitting patients at the acute stage of MI, 374 centers gave their consent to participate: 42 university hospitals, 225 public hospitals, 96 private clinics and 11 other centers. The final participation rate was 93% (n=39) for university hospitals, 59% (n=132) for public hospitals, 46% (n=44) for private clinics and 73% (n=8) for other centers.13
Patient SelectionAll consecutive patients admitted to the participating centers November 1-30, 2005 were included in the registry if they met the following criteria:
• Diagnosis of acute MI based on serum markers more than twice the upper limit of normal for creatine kinase, creatine kinase MB fraction, or troponins; and either chest pain lasting for at least 30min and not relieved by nitrates or electrocardiogram changes on at least 2 contiguous leads with pathological Q waves (at least 0.04s) or persisting ST-elevation or depression >0.1mV.
• Less than 48h from the beginning of symptoms to ICU admission.
• Informed consent for participation in the survey and follow-up.
Among the whole population, patients with at least one of the following criteria were defined as CHF patients (group 1):
• Previous CHF history before admission.
• Symptoms of CHF on admission according to the Framingham criteria.14
• Killip class≥2 on admission.
• Killip class≥2 at any time of hospitalization.
• Left ventricular ejection fraction (LVEF)≤40% at any time during hospitalization.
Patients with none of these criteria constituted patients without CHF (group 2).
Data CollectionFor each patient, cardiovascular history was recorded, along with current medications at the time of admission, risk factors (smoking status, history of hypertension or treated hypertension, cholesterol concentration >6.5 mmol/L or treated hyperlipidemia, family history, diabetes mellitus defined by treatment with insulin or oral antidiabetic medications, or previously documented fasting hyperglycaemia (>6.99 mmol/L)), in-hospital clinical course including maximum Killip class, initial diagnosis, treatment management, and LVEF, if assessed at any time during hospitalization. Where more than one method was used to assess LVEF, the value selected for analysis was chosen on the basis of the following priority ranking of assessment methods: a) left ventricular contrast angiography; b) radionuclide angiography, and c) echocardiography with quantitative or visual estimation of LVEF.
All medications at hospital discharge were recorded. Two groups of CHF patients were constituted: patients receiving both BB and antagonists of the renin-angiotensin-aldosterone system (RAAS) (either ACE inhibitors, angiotensin receptor blockers, or aldosterone blockers) and those receiving either none or only one of these classes of medications.
One-year follow-up was obtained for 99% of the patients.
Statistical AnalysisAll continuous variables, except time to admission, are reported as mean (standard deviation). All categorical variables are described in absolute and relative frequency distributions. Groups were compared by unpaired t tests for continuous variables and χ2 tests for discrete variables. Multiple logistic regression analysis for in-hospital mortality included: sex, age, administrative regions, type of centers and admission units, CHF, hypertension, diabetes, hypercholesterolemia, smoking, family history of coronary artery disease, history of stroke, peripheral arterial disease, renal insufficiency, cancer, coronary artery bypass graft and angioplasty, chronic obstructive lung disease, ST-elevation myocardial infarction (STEMI) and non-ST-elevation myocardial infarction (NSTEMI), systolic blood pressure, heart rate, and atrial fibrillation on admission, and medical classes of cardiovascular drugs administered before admission.
One-year survival was calculated using the Kaplan-Meier method, with the use of the log rank test to compare groups. Variables with a P value <.10 on Cox univariate analyses were entered into a Cox multivariate model complied with the proportional hazards assumption. To further assess the impact of prescribing recommended medications in CHF patients, a propensity score for receiving both RAAS antagonists and BB was generated using logistic regression analysis, and 2 cohorts of patients (one with and the other without both recommended classes of medications) were matched on the propensity score, permitting comparison of 1-year survival in groups of patients with similar baseline characteristics.
For all tests, a P<.05 was considered significant.
Results Baseline CharacteristicsThe whole population of the FAST-MI registry consisted of 3059 patients with STEMI or NSTEMI. Within this population, 1149 patients (37.6%) presented with at least one of the CHF criteria and constituted group 1. Categorization of patients according to the criteria was as follows: previous CHF history (n=173; 15.1%), symptoms of CHF on admission (n=365; 31.8%), Killip class≥2 on admission (n=700; 60.9%), Killip class≥2 at any time of the hospitalization (n=814; 70.8%), LVEF<40% (n=375; 32.6%). Symptoms of CHF on admission might be the most debatable criterion. However, only 20 patients (1.7%) were included based on this single criterion. Group 2 consisted of the remaining 1910 patients (62.4%) without any CHF criteria.
Baseline characteristics of both groups are presented in Table 1. Group 1 patients were older (P<.0001), less frequently male (P=.0001), and more often had previous history of hypertension, diabetes, and renal failure. On admission, mean heart rate was higher in group 1 than in group 2, with a higher percentage of patients with heart rate >100 bpm. Mean systolic pressure was lower in group 1 than in group 2, with a higher percentage of patients with systolic pressure <100mmHg. More group 1 patients had atrial fibrillation on admission compared with group 2 patients (P<.0001).
Table 1. Characteristics of Patients With (Group 1) or Without (Group 2) Heart Failure Criteria on Hospital Admission.
| Variable | Group 1 | Group 2 | P |
| Patients | 1149 (37.5) | 1910 (62.5) | <.0001 |
| Age, years | 72.7±13.3 | 63.5±13.9 | <.0001 |
| Male sex, % | 62.4 | 72 | <.0001 |
| Previous history of (%): | |||
| Hypertension | 65.9 | 52.2 | <.0001 |
| Diabetes | 30.5 | 19.6 | <.0001 |
| Renal failure | 9.5 | 2.8 | <.0001 |
| HR, bpm | 86±24 | 77±16 | <.0001 |
| Patients with HR>100 bpm, % | 27 | 8.9 | <.0001 |
| Mean SBP, mmHg | 137±31 | 140±27 | <.0020 |
| Pts with SBP<100 mmHg, % | 8.3 | 4.9 | <.0010 |
| Atrial fibrillation, % | 12 | 4 | <.0001 |
HR, heart rate; SBP, systolic blood pressure.
Values are expressed as no. (%) or mean±standard deviation, unless otherwise indicated.
In STEMI patients, primary percutaneous coronary interventions (PCI) were less frequently performed in group 1 patients (30.1%) than in group 2 patients (37.1%). Similarly, thrombolysis was less frequently prescribed in group 1 patients (pre-hospital, 15.3%; in-hospital, 9%) than in group 2 patients (pre-hospital, 20.6%; in-hospital, 11%). Overall, there was no revascularization in 45.6% of group 1 patients compared to 31.4% of group 2 patients (P<.0001). In revascularized CHF hospital survivors, 1-year survival remained lower than in patients without CHF (90.8% vs 96.2%; P<.001).
Medications at Hospital DischargeMedications prescribed in patients alive at discharge (n=2894; 1009 in group 1 and 1885 in group 2) are listed in Table 2. At discharge, all classical CHF treatment classes except beta-blockers were more often prescribed in group 1 than in group 2. Antiplatelet agents and statins were less frequently prescribed in group 1 than in group 2. Conversely, oral anticoagulants and, not surprisingly amiodarone, were more often prescribed in CHF patients than in patients without CHF.
Table 2. Medical Treatment Prescribed at Hospital Discharge to Patients With (Group 1) or Without (Group 2) Heart Failure Criteria.
| Group 1 | Group 2 | P | |
| Recommended drugs in heart failure | |||
| Beta-blockers, % | 66.2 | 76.1 | <.0001 |
| ACEI/ARB, % | 67.9 | 62.1 | <.0020 |
| Beta-blockers+ACEI/ARB, % | 50.1 | 52.1 | ns |
| Diuretics, % | 47.7 | 10.9 | <.0001 |
| Mean daily dosage of furosemide (n=446) * , mg/day | 55.5±76.8 [40] | 41.3±33.9 [40] | ns |
| Aldosterone-blockers, % | 11 | 0.9 | <.0001 |
| Digoxin, % | 1.1 | 0.3 | <.0100 |
| Other drugs, % | |||
| Acetylsalicylic acid | 83.4 | 87.9 | <.0010 |
| Clopidogrel | 67.5 | 79.4 | <.0001 |
| Statins | 72 | 82 | <.0001 |
| Oral anticoagulants | 1.4 | 0.6 | <.0300 |
| Calcium-blockers | 15.8 | 13.5 | ns |
| Nitrates | 17.7 | 16.6 | ns |
| Amiodarone | 14.9 | 3.4 | <.0001 |
ACEI, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blockers; ns, not statistically significant.
* Mean±standard deviation [median].
Patients receiving furosemide with a known daily dose at discharge were 446 (342 in group 1 and 102 in group 2). The daily dose varied between 10-750mg/day. The mean was not significantly higher in group 1 than in group 2 and the median was similar between both groups (40mg) (Table 2).
In-Hospital CourseIn-hospital mortality was dramatically increased in group 1 compared to group 2 (12.2% vs 1.3%; P<.0001). In addition, most in-hospital complications, including arrhythmias, re-infarction or bleeding complications, were more frequent in CHF patients (Table 3). Length of both ICU and total hospital stay was longer in group 1 than in group 2 (Table 3).
Table 3. Comparison of in-Hospital Mortality, Complications, and Duration of Stay Between Patients With (Group 1) and Without (Group 2) Heart Failure Criteria.
| Variable | Group 1 | Group 2 | P |
| Mortality | |||
| 5-day total mortality, % | 7.4 | 0.9 | <.0001 |
| In-hospital total mortality, % | 140 (12.2) | 25 (1.3) | <.0001 |
| Complications | |||
| Ventricular fibrillation | 42 (3.7) | 25 (1.3) | <.0001 |
| Sustainedventricular tachycardia | 47 (4.1) | 23 (1.2) | <.0001 |
| Atrial fibrillation | 112 (9.7) | 63 (3.3) | <.0001 |
| Reinfarction | 36 (3.1) | 20 (1) | <.0001 |
| Atrioventricular block | 34 (3) | 17 (0.9) | <.0001 |
| Stroke | 18 (1.6) | 11 (0.6) | .0060 |
| Major bleeding | 39 (3.4) | 28 (1.5) | <.0001 |
| Blood transfusion (any) | 84 (7.3) | 43 (2.3) | <.0001 |
| Duration of hospital stay | |||
| ICU stay, days | 6.5±6.9 | 4.3±3.7 | <.0010 |
| Total hospital stay, days | 12±10.8 | 7.6±6.2 | <.0001 |
ICU, intensive care unit.
Values are expressed as no. (%) or mean±standard deviation, unless otherwise indicated.
Multiple logistic regression analysis showed that CHF remained a major independent predictor of in-hospital death (odds ratio [OR]=5.80; 95% confidence interval [95%CI], 3.65-9.22; P<.001). Associated predictors of mortality were age (OR=1.05; 95%CI, 1.03-1.07; P<.0001), chronic obstructive lung disease (OR=1.92; 95%CI, 1.10-3.34; P=.021), family history (OR=0.46; 95%CI, 0.24-0.87; P=.018), systolic blood pressure on admission (OR=0.98; 95%CI, 0.97-0.99; P<.0001), heart rate on admission (OR=1.01; 95%CI, 1.00-1.02; P=.002), and presence of STEMI vs NSTEMI (OR=1.76; 95%CI, 1.24-2.51; P=.002).
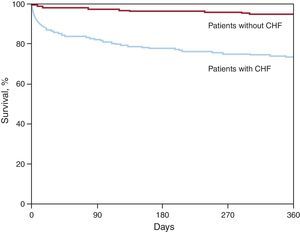
Long-Term Outcomes; Role of Recommended Medications at DischargeOne-year mortality was considerably higher in CHF patients (26.6% vs 5.2%; P<.0001) (Figure 1).
Figure 1. One-year survival of patients with (83.6%) or without (96%) heart failure (P<.0001). CHF, congestive heart failure.
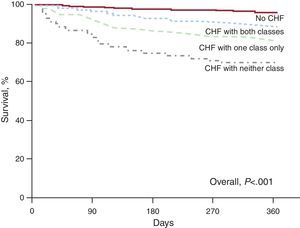
In hospital survivors, cumulative 1-year survival rate was the highest in patients without CHF (n=1885), while CHF patients receiving neither class of recommended medications (BB and ACE inhibitors) (n=127) (adjusted hazard ratio [aHR]=1.66; 95%CI, 1.08-2.55; P=.02) had the lowest survival rate. The CHF patients treated with both recommended medications (n=538) (aHR=1.16; 95%CI, 0.82-1.64; not statistically significant) had a better intermediate survival rate than CHF patients treated with either class (n=344) (aHR=1.47; 95%CI, 1.04-2.07; P=.03) (Figure 2). However, survival of patients receiving all recommended medications remained significantly poorer than survival of patients without CHF (P<.001).
Figure 2. Unadjusted cumulative 1-year survival curves comparing hospital survivors without heart failure (n=1885), heart failure patients treated by both beta-blockers and angiotensin-converting enzyme inhibitors (n=538), heart failure patients treated with either class (n=344), and heart failure patients receiving neither class (n=127). CHF, congestive heart failure.
In group 1 hospital survivors, 1-year survival was much better in CHF patients with preserved systolic function (LVEF≥50%; n=289) than in those with systolic CHF (LVEF<40%; n=375) (85.1% vs 68.2%, respectively; P<.001).
Cox multivariate analysis of hospital survivors (Table 4) showed that CHF was an independent correlate of 1-year mortality (OR=1.55; 95%CI, 1.10-2.17; P=.011). Other independent predictors were Global Registry of Acute Coronary Events (GRACE) score, age, concomitant illnesses, lack of reperfusion therapy in STEMI patients, use of PCI during hospital stay, early use of low molecular weight heparin. However, when discharge medications were added to the model, the co-prescription of BB and RAAS antagonists was associated with reduced mortality (OR=0.74; 95%CI, 0.56-0.99; P=.044), while the presence of CHF per se was no longer significant, bearing witness to the importance of these medications. After restricting multivariate analysis to CHF patients, and compared with patients receiving both classes of recommended medications, 1-year mortality was higher in those treated with 1 class only (OR=1.33; 95%CI, 0.89-1.98; P=.16) and significantly increased in those receiving none (OR=1.95; 95%CI, 1.16-3.29; P=.012).
Table 4. Results of Cox Multivariate Analysis in Hospital Survivors for Long-Term Outcomes.
| Variables | HR (95%CI) | P |
| Age | 1.03 (1.01-1.04) | .001 |
| Sex | 0.85 (0.64-1.13) | .263 |
| Systemic hypertension | 1.14 (0.82-1.58) | .426 |
| Diabetes | 1.53 (1.17-2.01) | .002 |
| History of stroke | 1.85 (1.26-2.71) | .002 |
| History of peripheral arterial disease | 1.11 (0.79-1.57) | .555 |
| History of renal insufficiency | 1.72 (1.20-2.45) | .003 |
| Chronic obstructive lung disease | 2.16 (1.42-2.27) | .000 |
| Presence of heart failure | 1.55 (1.10-2.17) | .011 |
| GRACE score | 1.01 (1-1.01) | .000 |
| Atrial fibrillation on admission | 1.17 (0.81-1.69) | .391 |
| Use of PCI during hospital stay | 0.44 (0.32-0.60) | .000 |
| Reperfusion therapy | ||
| STEMI with reperfusion (ref.) | .002 | |
| NSTEMI | 2.23 (1.37-3.61) | .001 |
| STEMI without reperfusion | 1.98 (1.20-3.27) | .007 |
| Early use of low molecular weight heparin | 0.69 (0.53-0.90) | .006 |
| Early use of anti-GpIIb/IIIa treatment | 0.86 (0.60-1.24) | .418 |
| Major bleeding | 1.81 (0.95-3.46) | .072 |
95%CI, 95% confidence interval; GPIIb/IIIa: glucoprotein IIb/IIIa; GRACE, Global Registry of Acute Coronary Events; HR, hazard ratio; NSTEMI, non ST-elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST-elevation myocardial infarction.
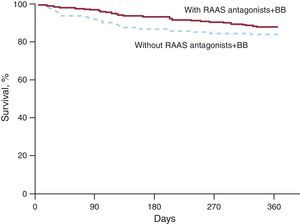
Likewise, when survival was compared in the 2 cohorts of patients matched on the propensity score for receiving both BB and RAAS antagonists (347 patients in each cohort), patients treated with both classes of medications had a definite trend to higher survival (88% vs 83%; P=.054), despite the fact that they had similar baseline characteristics (Table 5, Figure 3).
Table 5. Baseline Characteristics of the Patients With Heart Failure, Matched on a Propensity Score for Receiving Both Beta-Blockers and Antagonists of the Renin-Angiotensin-Aldosterone System.
| Variable | Patients without BB+RAAS antagonists (n=347) | Patients with BB+RAAS antagonists (n=347) | P |
| Age, years | 71±13 | 70±13 | .38 |
| Male sex | 222 (64) | 218 (63) | .75 |
| Previous history of | |||
| Hypertension | 225 (65) | 221 (64) | .75 |
| Diabetes | 108 (31) | 107 (31) | .93 |
| Renal failure | 30 (9) | 27 (8) | .68 |
| STEMI | 156 (45) | 159 (46) | .33 |
| GRACE score | 187±38 | 184±37 | .96 |
| Hospital stay, days | 12±11 | 12±8 | .82 |
BB, beta-blockers; GRACE, Global Registry of Acute Coronary Events; RAAS, renin-angiotensin-aldosterone system; STEMI: ST-elevation myocardial infarction.
Values are expressed as no. (%) or mean±standard deviation, unless otherwise indicated.
Figure 3. One-year survival in cohorts (n=347) matched on propensity score for receiving both beta-blocker and antagonists of the renin-angiotensin-aldosterone system. BB, beta-blockers; RAAS, renin-angiotensin-aldosterone system.
DiscussionOur results show that acute MI patients with CHF have less favorable baseline characteristics and much poorer outcome than patients without CHF. In particular, early mortality is close to 10-fold higher than in patients without CHF. Our registry also shows that medications such as BB or RAAS antagonists are insufficiently prescribed, in spite of the most recent recommendations.8, 15 Of note, as documented by both multivariate and propensity score analyses, CHF patients receiving both BB and RAAS antagonists at discharge had a significantly better 1-year survival than did patients not receiving these medications, and not significantly different from that of patients without CHF, once potential confounders were taken into account. In contrast, patients not receiving both classes of recommended medications remained at a significantly increased risk.
Early Outcome in Patients With Heart Failure in Acute Myocardial InfarctionAmong criteria used for defining CHF, symptoms of CHF according to the Framingham description14 could appear subjective. The study data came from a registry, so control of validity was not possible. However, few patients (n=20; 1.7%) were included on the basis of clinical symptoms only.
Mortality of acute MI is declining.16, 17, 18, 19 Although most of the recent population-based studies showed a temporal trend for a decline in incidence of CHF after MI,20, 21 this decline appears rather modest and balanced by some epidemiological studies reporting an increased incidence of CHF.22, 23 CHF remains a major risk factor for death after developing MI.24
Despite the fact that 6-month mortality in MI decreased during the last decade,16 contributing to rank cardiovascular diseases as the second cause of death in France behind cancer for the first time in 2004,25 it appears that the presence of CHF during the acute phase of MI still plays a major negative role in prognosis.
The increased mortality and morbidity in FAST-MI patients with CHF criteria confirms the deleterious effect of either pre-existing CHF or development of symptomatic left ventricular dysfunction at the acute phase of MI. The CHF patients presented with cumulative severity factors. Although these parameters had a definite impact on early mortality, presence of CHF remained an independent correlate of in-hospital death, with a nearly 10-fold increased risk after multivariate adjustment.
Use of Recommended MedicationsMedical classes of cardiovascular drugs usually prescribed in CHF patients were more often administered in group 1 patients, except for beta-blockers. International recommendations emphasize the major protective role of BB in both MI and CHF.8, 15 Percentages of prescription are, however, similar or slightly increased compared with previous registries,9, 16, 26 except for the GRACE registry where the rate of BB was higher.27 In CHF registries, prescription of BB appeared slightly lower than in CHF patients in the FAST-MI registry. Recent epidemiological studies also report a lower use of BB in MI patients with early-onset CHF.28, 29 This could be explained by the fact that physicians take into account the potential risk of developing cardiogenic shock associated with the early prescription of BB in patients with early signs of CHF at the acute phase of MI.30 In addition, low systolic arterial pressure is often the only reason for not prescribing BB.31
Long-Term Outcome in Hospital Survivors and Impact of Recommended MedicationsBeyond the acute stage, CHF patients remain at increased risk. Both BB and RAAS antagonists have been studied in this context. Meta-analyses of the use of ACE inhibitors in patients with MI and left ventricular systolic dysfunction have documented a significant reduction in cardiovascular events and mortality. In MI studies, this finding most likely reflected a beneficial effect of ACE inhibitors in patients who suffered larger infarcts.32 In our study, prescription rates of ACE inhibitors were similar to those of the previous acute coronary syndromes registries;9, 16, 26 however, they were slightly lower than those of CHF registries.33 The use of aldosterone antagonists was also a promising cardiovascular class in the field of myocardial infarction despite a lowerprescriptionpercentage.34 As regards BB, all early trials in acute MI patients excluded those with CHF. Only the CAPRICORN trial2 specifically addressed the impact of BB in acute MI patients with poor left ventricular function. As the CAPRICORN trial formally was a negative trial due to the change in primary criteria during the course of the study, registry data are important to further support current recommendations in this field. In our population, patients prescribed both BB and RAAS antagonists had improved outcomes when compared with CHF patients receiving none or only one of these classes of medications. The propensity score-matched populations reinforced the findings of the Cox multivariate analysis: combination of both classes decreased the risk of 1-year mortality by approximately 30%. Interestingly, after multivariate adjustment, although a trend persisted for increased mortality in CHF patients receiving both BB and RAAS antagonists compared with patients without CHF, this trend was not statistically significant, bearing witness to the considerable progress achieved in the management of acute MI patients with CHF.
LimitationsThere was an “overrepresentation” of academic institutions in the selection of the centers. However, 60% of community hospitals and nearly 50% of private clinics participated. Because there was a much larger number of community hospitals, a majority of the included patients came from these hospitals, while patients admitted to academic institutions represented about a quarter of the population.
All potential confounding co-variables may not have been recorded. All variables known to have the most important impact on outcomes, however, were available and allowed high-quality adjustments. All patients with CHF criteria were included in the CHF group, whether these criteria were present before the occurrence of MI or appeared during the ischemic episode. The choice of CHF criteria can be discussed. However, consistent with the definite presence of CHF, group 1 patients presented with lower systolic arterial pressure and higher heart rate, and very often received diuretics, including high-dose furosemide.
In the setting of CHF and a short hospital stay, medications at discharge could not reach the recommended final level of titration. Some CHF patients, because of their poor condition, could not quickly receive full therapy and could be discharged with a scheduled early outpatient visit in order to improve tolerance. For these reasons, some patients not receiving both BB and RAAS antagonists possibly were patients awaiting either introduction or titration of drugs during later outpatient visits. However, difficulties in titration could reflect bad hemodynamic adaptation, thereby suggesting a higher degree of severity of the constituted ischemic cardiomyopathy. Finally, even in this case, the prognostic information delivered by the highest-risk treatment group (Figure 3) remained appropriate.
ConclusionsEven in the current era, patients admitted for acute MI with CHF criteria are still at very high risk of mortality. Our data are concordant with the favorable effects on mortality documented in randomized trials for recommended medications. They appear especially important as, other than the CAPRICORN trial, no other controlled trial data are available for BB used at the acute stage of MI in patients with CHF. Special efforts should be made to encourage the prescription of recommended medications in acute MI patients with either a past or current history of CHF.
FundingThe FAST-MI Registry is a registry of the French Society of Cardiology, supported by unrestricted grants from Pfizer and Servier. Additional support was obtained from a research grant from the French institution CNAM (Caisse Nationale d’Assurance Maladie).
Conflicts of interestDr. Juillière has received research grants from AstraZeneca, and consulting and/or lecture fees from Abbott Vascular, AstraZeneca, Bristol-Myers-Squibb, Novartis, Sanofi-Aventis, and MSD-Schering-Plough for participation to investigational trials as French national coordinator and/or to meetings as speaker. Dr. Galinier has received consulting fees and support for travel from Pfizer and Servier. Dr. Simon has received unrestricted research grants from Pfizer and Servier, and also fees for consulting or for sponsored symposia from AstraZeneca, Lilly, Sanofi-Aventis, Bristol-Myers-Squibb, Daichi-Sankyo, and Bayer-Schering. Dr. Danchin has received research grants from Pfizer and Servier for the study, as well as consulting fees and support for travel from Servier. He has also received research grants from Astra-Zeneca, Eli-Lilly, GlaxoSmithKline, Merck, Novartis, Sanofi-Aventis, and The Medicines Company, and lecture and/or consulting fees from AstraZeneca, Bristol-Myers Squibb, Boehringer-Ingelheim, GlaxoSmithKline, Eli-Lilly, Menarini, Merck, Novartis, Novo, Sanofi-Aventis, and The Medicines Company. No other authors reported disclosures.
Received 27 July 2011
Accepted 29 October 2011
Corresponding author: Département de Cardiologie, Institut Lorrain du Coeur et des Vaisseaux, Allée du Morvan, 54500 Vandoeuvre-les-Nancy, France. y.juilliere@chu-nancy.fr