If you surf the net to check tctmd.com or europcr. com, or the many other competing web sites, looking for talks on bifurcations, you will notice that they always start showing a tree branch or 2 rivers slowly merging and always finish saying that you have to use one stent only and cover the side-branch origin. Hopefully, next year they will start with bull horns instead, a Spanish national obsession, because the study by Medina et al in this issue of Revista Española de Cardiología challenges these traditional concepts and proposes new solutions.1 The authors are among the first "amateurs" of intravascular ultrasound imaging, having applied it since the early 90s in their daily clinical routine2 but still able to look with fresh eyes at the images and transform their practice based on the results.
Alfonso Medina gained a worldwide reputation among the bifurcation enthusiasts for having saved them from the Babel Tower of the existing classifications. He introduced a logical sequence based on a simple concept which has become the new "esperanto" in the description of bifurcations.3
Now he and his coauthors propose a single stent partially covering the ostium of the left circumflex artery (LCx) ("floating") to treat the most difficult bifurcation, the bifurcation of the left main when the lesion is limited to the ostium of the left anterior descending (LAD) coronary artery.
The first theorem of the provisional stent law4 states that, if no plaque is present on the side branch on the wall opposite to the carina, ostium narrowings of the side-branch after stent implantation in the main vessel can promptly be reverted by kissing-balloon dilatation because the carina is always plaque free. The second theorem states that the plaque always extends into the bifurcation, which requires mandatory stent implantation across the side-branch. The technique applied in this study contradicts both theorems. Medina et al observed in a consecutive series that only a few lesions (19/71, 26.7%) have "vulnerable" carina anatomy at risk of malignant shift towards the side-branch. They identified with intravascular ultrasound (IVUS) a specific feature called "eyebrow sign" able to predict ostial compromise in 92.9% (13/14) of the cases. Is this a true novel finding adding useful information to guide our interventions?
Previous papers showed that a shallow angle between LCx and LAD is predictive of LCx ostium impairment after stenting, with a cut-off of 70 degrees.5 Atherectomy before stenting was proposed at the time for lesions with shallow angles.6 In this paper, there is an obvious preselection towards open angles between LAD and LCx because the average, both in the 19 patients with LCx ostial impairment after stenting and in the 52 without, is far above 70 degrees, 88.6, and 96.9 degrees, respectively.1 Even if the difference does not reach statistical significance, the angle is 10 degrees smaller on average in patients with compromise. The method used for the assessment of the bifurcation angle is not reported but it is likely based on the new quantitative coronary angiography programs for bifurcations.7 These programs have important limitations: the first is that the angle is normally measured only in one projection, often the projection that better shows the eccentric LAD lesion and not necessarily the projection with the wider angle between LAD and LCx. Even if we consider, however, techniques using biplane or rotational angiographic acquisition or true tomographic techniques such as multislice computed tomography (MSCT), the question is how we identify the direction of the 2 lines enclosing the angle. Figure 4A of Medina et al1 gives a good example of the problem. The quantitative coronary angiography software is likely to prolong and straighten the lines into the LAD and LCx but you may then overestimate the true angle at the level of the bifurcation which is very shallow at the origin, the only position which counts for the carina shift. In Figure 5A, angiography is probably deceitful because there is superimposition of the 2 vessels in the very proximal segment, covering the 2-3 mm where the vessels run nearly parallel. The IVUS "eyebrow sign" solves these angiographic limitations and better identifies the risk that enlarging the LAD ostium will significantly shift the carina towards the LCx.
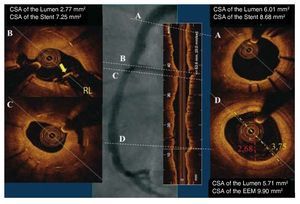
Figure.Light-lab C7 DragonFly catheter pulled back for 55 mm in a long in-stent restenosis 2 years after deployment of 2 Yukon (sirolimus eluting, no polymer) stents in the right coronary artery. In panel B an arrowhead indicates an uncovered struts at the level of the bifurcation of a right ventricular branch despite the massive hyperplasia on the opposite wall and in the cross-section immediately below (panel C). Please note mild hyperplasia in the proximal stent (A) and the preserved lumen in the distal reference segment (D). Despite the concentric fibrotic intimal thickening, the increased penetration -which is, together with the rapid acquisition, one of the main advantages of frequency domain optical coherence tomography- allows complete delineation of the external elastic membrane. CSA indicates cross-sectional area; EEM, external elastic membrane; SB, sidebranch.
Why bother to identify the risk of LCx ostium compromise when this is minor (<50% diameter stenosis [DS]) in most cases (10/19, 52.6%) and also when it is >50% DS there is no real functional flow impairment (55.6%), as shown by the results of the pressure wire study? There is an obvious anguish in leaving a moderate to severe stenosis at the ostium of a main epicardial vessel, or applying plain balloon angioplasty with no drug elution to reduce the hyperplastic response and the recoil. The patient of Figure 4A-B (Medina et al1) had a significant plaque accumulation in the distal left main, opposite to the origin of the LCx, and a straight line across the first segment of the LCx delineates with the LAD centerline an angle which is at most 45 degrees. When these signs and the "eyebrow sign" are neglected, a result like the angiogram of Figure 3 (Medina et al1), left lower panel, can be expected, correctable only with a second stent to the LCx, difficult to advance at this stage and leaving a partially crushed LAD stent.
Is the floating stent technique going to replace provisional stenting of the main vessel across the side-branch ostium and all the 2-stents techniques for all Medina 0,1,0 and 0,0,1 bifurcations, wherever located along the coronary tree? Very unlikely for many reasons. MSCT shows that the left main bifurcation has far wider angles between its daughter vessels than bifurcations of the LAD-diagonal or LCx-marginal branch, which means most of these side-branches will develop significant ostial compromise even in the absence of pre-existing ostial stenoses.8
Even if angiographically the lesion appears to spare the proximal distal vessel, there is almost invariably a plaque opposite to the flow divider along the entire bifurcation. For the left main, the vessel is so large that a minor uncovered residual lesion or even a small flap at the proximal edge is not a concern but this is different in smaller vessels or when there is greater chance that kissing balloon must be applied, severely damaging the plaque proximally. Similarly, some level of plaque accumulation at the ostium of the side-branch opposite to the flow divider poses a risk of disruption after kissing balloon, which can be prevented by stenting across the sidebranch and displacing the struts of the main vessel stent to partially cover the side-branch ostium. Finally, precise positioning is already very difficult for a large proximal vessel such as the left main. Even with an angiographic acquisition of the IVUS catheter at the end of the carina the protrusion of the stent into the ostium is major, more than 2 mm, sufficient to cover almost completely the ostium of most sidebranches along the coronary tree, thus defeating the purpose of the use of a floating stent, which is to leave free access to the sidebranch. The authors appear very relaxed about leaving uncovered struts floating in the middle of the LCx ostium. The presence of a circumferential coverage documented with optical coherence tomography (Figure 6B of the original manuscript) is more reassuring than seeing still bare struts at follow-up but the redundant tissue observed, probably reflecting organized thrombus and hopefully reendothelialised after so many months,9 is far less pleasant than the usually finer line of tissue coverage present above apposed struts. An extreme example of discrepancy between excessive circumferential intimal growth of the stents apposed to the wall and total lack of coverage of a strut across the origin of a right ventricular branch is shown in Figure 1.
In summary, we think Medina et al1 describe a rational approach with the mandatory use of IVUS to improve results of a technique too often used inappropriately in the past. If IVUS shows true right angles with the LCx (no "eyebrow sign"), LAD plaque is mainly limited to the ostium without involving the distal left main, and the LCx ostium has no stenoses (or better yet, IVUS shows no significant plaque accumulation), a floating stent technique can be considered. Filming the IVUS catheter position at the time of the last frame showing the carina can help to position the stent, but the systo-diastolic movement, an oblique angle of the imaging plane due to a non coaxial alignment of the IVUS catheter, and the fear to avoid incomplete ostium coverage mostly lead to an unavoidable excessive stent protrusion, apparently benign at follow-up.
SEE ARTICLE ON PAGES 1240-9
Correspondence: Prof. C. Di Mario,
Consultant Cardiologist, Royal Brompton Hospital, Sydney St. London SW3 3NP, United Kingdom
E-mail: C.DiMario@rbht.nhs.uk