To the Editor,
Implantable cardioverter defibrillators (ICDs) are an established therapy for prevention of sudden death in an adult patient.1 However, the implantation of these devices in paediatric patients with potential risk of sudden death (SD) has many disadvantages, and there is little information on implant use and methodology for this population.2
Despite technological development, intravenous defibrillator implantation in children and infants poses multiple challenges.3 There are no electrodes modified for their small vessel diameter, with the consequent risk of venous thrombosis, nor devices adapted to their body surface. If we add to this physical activity and growth, as well as a greater incidence of infections, the risk of complications after implantation is not negligible.4 Therefore, an extracavitary method of implantation has been postulated as the technique of choice in the paediatric population. Many versions of the extracardiac implant technique have been reported, but there is still no agreed procedure.
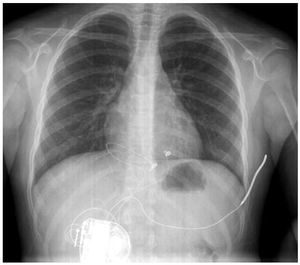
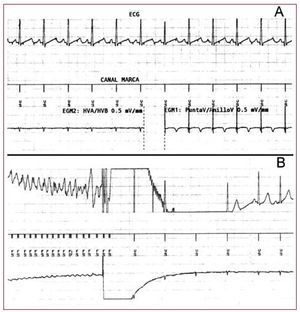
We report the case of a 7-years-old girl with a history of sudden death in several immediate family members (mother and 2 brothers), who was diagnosed with channelopathy and underwent implantation of a defibrillator in July 2007 in our centre.The following surgical protocol was followed: a subxiphoid window was performed under general anaesthesia to access the front of the right ventricle where 2 epicardial unipolar probes were implanted (Medtronic 5071-35 cm/Medtronic 5071-35 cm) for detection and stimulation, converted in a bipolar IS-1 connector through an adapter. Furthermore, a defibrillation coil (Medtronic transvenous 6937, 58 cm) was tunnelled subcutaneously from the subxiphoid window towards the left armpit. All the probes were connected to a generator (Medtronic Entrust D154VRC) which was housed in a retrorectal abdominal pouch below the right costal margin (Figure 1). The correct sensing and pacing thresholds were verified after the implantation, along with the correct impedance of the probes. Furthermore, the correct defibrillation shock of 21J was found after inducing ventricular fibrillation by a shock on the T wave (Figure 2).
Figure 1. Chest x-ray in posteroanterior view. The arrangement of the epicardial electrodes and the subcutaneous coil, as well as the generator housing, are shown.
Figure 2. A: shows the surface electrocardiogram (DI lead), the device channel trace and electrogram obtained between HVA and HVB (morphology channel) on the left, and the electrogram obtained from the epicardial electrodes (sensing and stimulation) on the right. B: shows the electrocardiographic records, trace channel and morphology electrogram during induced ventricular fibrillation in the operating room, reversed by a shock of 25 J from the device.
After the procedure, the patient was admitted to the paediatric intensive care unit for 24 h with a good clinical outcome without complications from the procedure. Oral tolerance began at 24 h without abdominal discomfort. During the first 24 h after implantation, prophylactic antibiotics (cefazolin 30 mg/kg/8 h) were given.
After hospital discharge, the patient was referred for outpatient control and monitoring in the defibrillator consultation of paediatric cardiology. During follow-up (a year and a half), with regular checks every 6 months, no significant arrhythmias were detected. The sensing and stimulation thresholds, as well as impedances, remained stable. The patient has remained asymptomatic without any kind of limitation placed on physical activity. The device is programmed with bradycardia therapy in VVI mode with a lower frequency limit of 40 beats/min and with a single ventricular fibrillation detection zone with frequencies above 250 beats/min with maximum energy shocks.
The implantation of a subcutaneous coil, designed for intravenous use, reduces the number of complications of probe vascular access in children. Use of the implantation technique can prevent venous obstruction, adhesions in venous walls, cardiac cavities or valvular structures, tricuspid regurgitation, the tension on the tube with the growth of the child and future hypothetical intracavitary abandonment if the probe is damaged and cannot be extracted. Furthermore, access via subxiphoid implant for the epicardial probes avoids sternotomy or thoracotomy.
As a result, the challenge of implanting these devices in the paediatric population has led us to develop the technique described. It is minimally invasive and its safety and efficacy have been established after a year and a half of monitoring.