Most long-term ventricular assist devices (VADs) that are currently implanted are intracorporeal continuous-flow devices. Their main limitations include their high cost and inability to provide biventricular support. The aim of this study was to describe the results of using paracorporeal pulsatile-flow VADs as a bridge to transplant (BTT) in adult patients.
MethodsRetrospective analysis of the characteristics, complications, and outcomes of a single-center case series of consecutive patients treated with the EXCOR VAD as BTT between 2009 and 2015.
ResultsDuring the study period, 25 VADs were implanted, 6 of them biventricular. Ventricular assist devices were indicated directly as a BTT in 12 patients and as a bridge to decision in 13 due to the presence of potentially reversible contraindications or chance of heart function recovery. Twenty patients (80%) were successfully bridged to heart transplant after a median of 112 days (range, 8-239). The main complications included infectious (52% of patients), neurological events (32%, half of them fatal), bleeding (28%), and VAD malfunction requiring component replacement (28%).
ConclusionsEighty percent of patients with the EXCOR VAD as BTT achieved the goal after an average of almost 4 months of support. The most frequent complications were infectious, and the most severe were neurological. In our enivonment, the use of these pulsatile-flow VAD as BTT is a feasible strategy that obtains similar outcomes to those of intracorporeal continuous-flow devices.
Keywords
Despite the continuing advances in therapy for heart failure (HF), a large number of HF patients eventually have HF refractory to conventional treatments. For most of these patients, cardiac transplant (CTx) remains the treatment of choice. Nonetheless, because of the limited number of donors and the presence of contraindications or comorbidities, many patients do not have access to this therapeutic option. Consequently, ventricular assist devices (VADs) have undergone rapid development in the last few years. These devices aim to provide circulatory support as a bridge to transplant (BTT) in patients with advanced HF waiting for a CTx, to provide support until cardiac function recovers in patients with reversible heart diseases (bridge to recovery), or as the definitive treatment in patients who are not candidates for CTx (destination therapy).
Currently, pulsatile-flow paracorporeal VADs have given way to a new generation of smaller, longer-lasting continuous-flow pumps that are placed by intracorporeal implantation. The results with these devices are very acceptable, although they have the limitations of high cost and difficulty in providing biventricular support. In Spain, where the mean waiting time for a CTx is less than 6 months (shorter than in neighboring countries) and where economic constraints hamper the expansion of these therapies, pulsatile-flow VADs may retain their usefulness as BTT.
The limited published evidence on the usefulness of long-term pulsatile VADs in adults is mainly based on isolated clinical cases.1–3 The aim of this study was to analyze the overall outcome of a strategy involving implantation of a pulsatile VAD of lengthy duration, the EXCOR device (Berlin Heart), for the purpose of BTT in a single-center series of adults with advanced HF.
METHODSStudy DesignThis is a retrospective, observational study based on a local registry, including all patients who received an EXCOR VAD between 2009 and 2015. The registry contains all the variables included in the IMACS registry (International Society for Heart and Lung Transplantation Registry for Mechanically Assisted Circulatory Support) and several additional variables considered to be of interest. The information recorded comprised the patients’ demographic, clinical, analytical, echocardiographic, and hemodynamic characteristics, the implantation data, and the follow-up data at 1 week, 1 month, and every 3 months thereafter. All VAD-related adverse events were specifically recorded: bleeding, thrombosis, stroke, infection, arrhythmia, right ventricular (RV) failure, VAD dysfunction, and sensitization due to the development of anti-HLA (human leukocyte antigen) antibodies. The primary objective of the study was to assess the usefulness of the VAD to help patients reach the final outcome (CTx or explantation due to improvement). The secondary aim was to analyze the complications that occurred during the period of circulatory support.
Patients and ProcedureVentricular assist device implantation was indicated in patients at least 16 years of age with chronic or acute HF, in New York Heart Association functional class IV, refractory to other treatment modalities, and included or being evaluated for inclusion on the CTx waiting list, but deemed to be unable to reach CTx without a bridging device. In general, they were patients hospitalized for HF on multiple occasions and requiring intravenous inotropic therapy. The patient's heart disease had to have anatomic characteristics favorable for proper functioning of the device: basically, severe left ventricular systolic dysfunction with a normal or increased chamber size. Hypertrophic and restrictive cardiomyopathies, and the presence of a previous sternotomy were considered unfavorable conditions.
All patients underwent right catheterization during their prognostic evaluation before VAD implantation. Patients with pulmonary arterial hypertension contraindicating CTx (≥ 2 of the following criteria: systolic pulmonary arterial pressure ≥ 50mmHg, transpulmonary gradient ≥ 15mmHg, pulmonary vascular resistance ≥ 3.5 Wood units) underwent a second catheterization procedure following administration of diuretics and inotropes (milrinone, levosimendan), systemic vasodilators (nitroprusside) and pulmonary dilators (inhaled nitric oxide). If values compatible with CTx were not reached and the patient was clinically stable, sildenafil or bosentan for 12 to 16 weeks was prescribed and right catheterization was repeated. Refractory pulmonary hypertension was established when the patient did not respond sufficiently to all these measures.
The cases were presented at a session of physicians and surgeons, who decided on the indication, the date of VAD implantation, and the choice of a left ventricular device or biventricular device, depending on the risk of RV failure after implantation.4 All patients were informed of the potential benefits and risks of the procedure, and all signed a specific informed consent document.
Beating heart surgery was performed through a sternotomy under extracorporeal circulation. During the procedure, patients were monitored with a Swan-Ganz catheter and transesophageal echocardiography to assess RV function and proper positioning of the cannulas.
During the first few hours following implantation, patients received vasoactive and inotropic agents to maintain a mean blood pressure of 70 to 80mmHg. In cases of left VAD implantation with RV failure, nitric oxide, milrinone, intravenous sildenafil, and/or inhaled prostacyclin derivatives were added. Once these drugs were withdrawn, HF treatment was initiated according to clinical practice guidelines. In patients with persistent RV failure, oral sildenafil was maintained.
If patients showed no significant bleeding, anticoagulation was started at 12 to 24hours following surgery with enoxaparin or sodium heparin, and was later changed to acenocoumarol with a target INR (international normalized ratio) of 2.5 to 3.5. At 2 to 3 postoperative days or once the drains had been removed, antiplatelet therapy (aspirin plus dipyridamole) was added, and its effect was determined using the Multiplate test (Roche Diagnostics, Basel, Switzerland). Aspirin resistance was established on Multiplate ASPI test values > 50 U and dipyridamole resistance on Multiplate ADP test values > 40 U. In these cases, the drug dose associated with resistance was increased. If resistance persisted, the drug was replaced by another antiplatelet agent, usually clopidogrel.
Management of the cannula orifices was carried out by specialized nursing staff according to the local protocol, using saline and chlorhexidine every 12hours during the first 3 days (and for local infection), and every 24 to 48hours thereafter. All patients and at least 1 of their family members underwent training to continue cannula maintenance, control anticoagulation (CoaguChek XS, Roche), and recognize the main device-related alarms at home following hospital discharge. The VAD pumps, which have transparent walls, must be examined 3 times daily to rule out the presence of thrombi in the interior.
Following discharge, patients attended follow-up visits every 7 to 15 days. Those who remained stable underwent another evaluation at the third month following device implantation to reassess the CTx indication (VAD as a bridge to decision) or to reactivate them on the waiting list, if they had been on the list previously (VAD as BTT). These patients were directly placed on the National Transplant Organization's waiting list as priority grade 1 (regional priority). If the circulatory support showed severe dysfunction, the patient could be included as grade 0 (national priority).
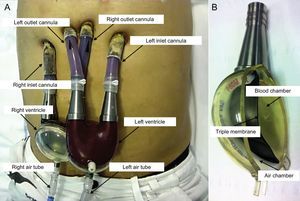
Ventricular Assist DeviceThe Berlin Heart EXCOR pulsatile-flow VAD (Berlin, Germany) consists of an inlet cannula, which is usually inserted in the apex of the left ventricle, and an outlet cannula, inserted in the ascending aorta (left support) or the right atrium and pulmonary artery (right support); bivalvular support can also be provided. The silicone cannulas are tunneled to a position above the diaphragm and exit through the skin. They are connected to a pump or artificial ventricle placed in a paracorporeal position at the upper hemiabdomen. The pumps have a transparent polyurethane outer casing, a variable size (10-80mL) and are divided by a triple membrane into 2 chambers (blood and air). The blood chamber, whose interior is coated with biocompatible material, communicates with the inlet and outlet cannulas through unidirectional valves. These were initially mechanical valves (single disc), but were later changed to polyurethane valves (3 leaflets), which produce less noise. The air chamber fills by positive pressure in systole and empties by negative pressure in diastole through an air tube connected to a pneumatic drive unit, which can be stationary (IKUS console) or portable for ambulatory use (Figure 1 and video 1 of the supplementary material).
Statistical AnalysisNumerical variables are reported as the mean ± standard deviation or the median and range, according to whether or not they met the assumption of normality. Categorical values are expressed as the absolute and relative frequency. Overall survival was estimated using the Kaplan-Meier actuarial method. The cumulative incidence function was estimated to analyze the time to transplant, because death acts as a competing event in the observation. The results are presented with 95% confidence intervals. All tests were 2-tailed, and statistical significance was established at P < .05. STATA/IC 14.1 was used for the statistical analyses.
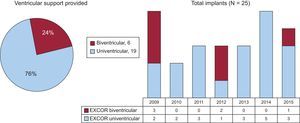
RESULTSBaseline Patient CharacteristicsBetween 2009 and 2015, 25 EXCOR VAD were implanted in Hospital Universitario Puerta de Hierro: 19 left ventricular and 6 biventricular (Figure 2). At the time of implantation, 12 patients had been on the CTx waiting list for a median of 37 days (range, 8-322). The other 13 patients underwent implantation as a bridge to decision: 9 because of a potentially reversible contraindication (refractory pulmonary hypertension in 7) and 4 because of a potential for HF improvement. The remaining characteristics are shown in Table 1.
Baseline Clinical, Echocardiographic and Hemodynamic Characteristics of the Population (n = 25)
| Women | 3 (12) |
| Age, y | 47.7 ± 11.8 |
| Body surface area, m2 | 1.9 ± 0.2 |
| Type of heart disease | |
| Nonischemic dilated cardiomyopathy | 9 (36) |
| Ischemic dilated cardiomyopathy | 8 (32) |
| Acute coronary syndrome | 3 (12) |
| Acute myocarditis | 3 (12) |
| Hypertrophic cardiomyopathy | 1 (4) |
| Arrhythmogenic RV cardiomyopathy | 1 (4) |
| INTERMACS grade | |
| 1-2 | 9 (36) |
| 3-4 | 16 (64) |
| Time since the HF diagnosis | |
| < 1 month | 3 (12) |
| 1 month-1 year | 3 (12) |
| > 1 year | 19 (76) |
| Previous cardiac surgery | 1 (4) |
| ICD user | 18 (72) |
| CRT device user | 8 (32) |
| Situation 48 h before implantation | |
| Inotropic agents | 21 (84) |
| Intra-aortic counterpulsation balloon | 8 (32) |
| Other circulatory support | 2 (8) |
| Respirator | 5 (20) |
| Dialysis | 1 (4) |
| Analytic parameters 24 h before implantation | |
| Creatinine, mg/dL | 1.4 ± 0.5 |
| GOT/AST, U/L | 23 [17-31] |
| Bilirubin, mg/dL | 1.5 ± 0.8 |
| Lactic acid, mg/L | 1.4 ± 0.8 |
| NT-proBNP, pg/mL | 8580 [6123-21 814] |
| Echocardiographic parameters | |
| LVED, cm | 6.7 ± 0.9 |
| LVEF, % | 20.4 ± 6.7 |
| Mitral regurgitation, IV | 12 (48) |
| Tricuspid regurgitation, III-IV | 12 (48) |
| Aortic regurgitation, III-IV | 1 (4) |
| TAPSE, mm | 15.7 ± 5.1 |
| Hemodynamic parameters | |
| CI, L/min/m2 | 2.2 ± 0.5 |
| PCP, mmHg | 26.9 ± 7.3 |
| sPAP, mmHg | 56.1 ± 18.5 |
| mPAP, mmHg | 39.8 ± 11.9 |
| mTPG, mmHg | 12.8 ± 7.5 |
| PVR, UW | 2.2 ± 2.1 |
| CVP, mmHg | 14.6 ± 6.6 |
| RVSWI,* mmHg × L/m2 | 0.71 ± 0.36 |
| Contraindication for CTx | 9 (36) |
| Refractory PAH | 7 (28) |
| Excess weight | 1 (4) |
| Severe failure of other organs | 1 (4) |
CI, cardiac index; CRT, cardiac resynchronization therapy; CTx, cardiac transplantation; CVP, central venous pressure; ICD, implantable cardioverter defibrillator; GOT/AST, glutamic-oxaloacetic transaminase/aspartate aminotransferase; HF, heart failure; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LVED, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; mPAP, mean pulmonary artery pressure; mTPG, mean transpulmonary pressure gradient; NT-proBNP, aminoterminal fraction of the brain natriuretic propeptide; PAH, pulmonary arterial hypertension; PCP, pulmonary capillary pressure; PVR, pulmonary vascular resistance; RV, right ventricular; RVSWI, right ventricular stroke work index; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
Unless otherwise indicated, the data express no. (%), mean ± standard deviation, or median [interquartile range].
The 16 VAD implanted between 2009 and 2013 had mechanical valves, whereas the 9 implanted from 2014 onward had polyurethane valves. Concomitant surgery was required in 2 patients: aortic valve plasty due to severe aortic insufficiency and patch repair of a left ventricular aneurysmal wall tear. Five patients (20%) had to undergo a new intervention within the first few hours: 3 because of uncontrolled bleeding, 1 to implant a short-term right support device, and 1 to correct a poorly positioned cannula.
Outcome During HospitalizationFour of the 25 patients (16%) receiving a VAD died of surgical complications. Of the 21 remaining patients, 18 (86%) were discharged and 3 remained hospitalized: 2 because they lived far from the hospital and 1 as a personal decision.
In the first 12 patients, antiplatelet therapy consisted of 200mg of aspirin and 300 to 800mg of dipyridamole. Subsequently, both drugs were replaced by clopidogrel 75mg daily due to poor digestive tolerance to dipyridamole and laboratory evidence of a lack of effectiveness.
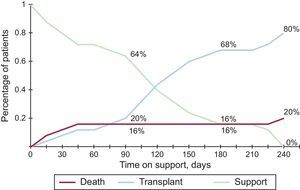
Final OutcomeTwenty of the 25 patients (80%) in the series reached CTx following a median of 112 days (range, 8-239) of support. The other 5 (4 left and 1 biventricular) died after a median of 17 days (range, 6-218). Survival at 30 days was 88% (95% confidence interval, 66-96). The competing risk curve showing the patients’ situation at 90, 180 and 240 days after VAD implantation is shown in Figure 3.
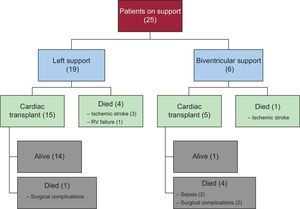
Overall 1-year survival of the transplanted patients (15 left and 5 biventricular) was 75%, similar to that of transplanted patients without VAD support. Post-transplant mortality was higher in patients who had received a biventricular VAD (80% biventricular vs 7% left) and was higher at the beginning of the series. A flow chart showing progression of the patients through the study, specifying the type of VAD and outcome, is depicted in Figure 4.
In the 7 patients undergoing VAD implantation for refractory pulmonary hypertension, there was a marked reduction in pulmonary artery pressure that enabled them to be included on the CTx waiting list at a mean of 7.7 weeks. Univentricular support was used in all patients, and all patients underwent successful CTx, without RV failure. At 1 year post-transplant, all patients had a favorable outcome.
Complications Associated With Circulatory SupportTwenty-three patients (92%) experienced some type of adverse event with circulatory support (Table 2), the most common being infections (52% of patients). Most infections were bacterial cellulitis (20%) occurring around the cannula orifice, and all were controlled with local treatment and antibiotics. In addition, there were 3 abdominal infections (2 cholecystitis and 1 appendicitis) requiring surgery.
Adverse Events (n = 25)
| Patients with an event, no. (%) | Events | Event rate (patient-month)a | |
|---|---|---|---|
| Infection | |||
| Circulatory support-related | 5 (20) | 5 | 0.06 |
| Othersb | 8 (32) | 9 | 0.10 |
| Severe bleeding | |||
| Surgical (reintervention) | 3 (12) | 3 | 0.03 |
| > 48 h after surgeryc | 4 (16) | 4 | 0.04 |
| Stroke | |||
| Ischemicd | 5 (20) | 5 | 0.06 |
| Hemmorrhagic | 2 (8) | 2 | 0.02 |
| TIA | 1 (4) | 1 | 0.01 |
| Support change | |||
| Thrombosis | 4 (16) | 4 | 0.04 |
| Mechanical dysfunctione | 3 (12) | 3 | 0.03 |
| Right ventricular failure | |||
| Severed | 1 (4) | 1 | 0.01 |
| Moderate | 1 (4) | 1 | 0.01 |
| Mild | 3 (12) | 3 | 0.03 |
| New significant arrhythmia | |||
| Atrial | 3 (12) | 3 | 0.03 |
| Ventricular | 4 (16) | 4 | 0.04 |
| New anti-HLA sensitization | 1 (4) | 1 | 0.01 |
HLA, human leukocyte antigen; TIA, transient ischemic attack; VAD, ventricular assist device.
Seven patients (28%) had bleeding events (none of which were fatal) requiring transfusion: 3 were cases of postoperative bleeding and 4 were unrelated to the procedure (1 hemoperitoneum following spontaneous spleen rupture, 2 gastrointestinal bleeding, and 1 epistaxis).
The most severe complication was stroke, which occurred in 8 patients (32%). Five patients had ischemic stroke, and 4 of these cases were extensive and fatal (3 occurred in the first few days after surgery and 1 at 218 days). Two patients had hemorrhagic stroke (clinically mild and controlled by temporary withdrawal of anticoagulation) and 1 patient had a transient ischemic attack coinciding with a low INR. There were no significant differences in this complication related to the type of valve (mechanical or polyurethane) on the device (P = .31).
Seven patients (28%) needed replacement of the artificial ventricle, in 4 patients because of thrombi within the blood chamber, in 2 because of tears in the membrane layers separating the 2 chambers2 and in 1 due to rupture of the air cannula (Figure 5). In 10 other patients, small fibrin deposits were seen within the device, which were resolved by changing the antiplatelet/anticoagulation regimen, without requiring device replacement.
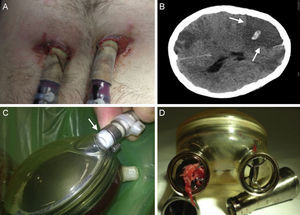
Complications in patients with circulatory support. A: Infection around the cannulas. B: Computed tomography of the brain showing a right ischemic stroke with central hemorrhagic transformation (arrows). C: Partial rupture at the connection of the air cannula to the artificial ventricle (arrow). D: Thrombus in the mechanical outflow valve of the artificial right ventricle.
Five patients (20%) had some degree of RV failure following implantation of a left VAD. Only 1 patient required right support of short duration (Levitronix CentriMag) due to severe RV failure.
Seven patients (28%) experienced arrhythmia with the VAD (3 atrial and 4 ventricular). Although tolerance to arrhythmia was good because 1 or 2 ventricles had support (video 2 of the supplementary material), attempts were made in all patients to control the heart rhythm with medication (4 patients) and/or electrical cardioversion (3 patients).
One patient who received various transfusions developed class II anti-HLA antibodies, which did not interfere with the posterior outcome of CTx.
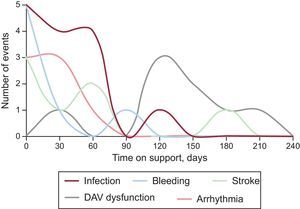
Most complications occurred in the first few weeks, with the exception of VAD dysfunction, which was more common after the third month (Figure 6).
DISCUSSIONThis study describes the largest series of patients treated with a long-term pulsatile-flow VAD in Spain, where the time on the CTx waiting list was relatively short (median 27 days in urgent cases and 120 days in elective procedures). A high percentage of the patients (80%) received adequate circulatory support until successfully reaching CTx. In total, 24% had biventricular support, which would not have been possible with the use of a continuous-flow VAD.
There are few scientific data on the use of pulsatile-flow VADs for BTT. In the case of the EXCOR device, most of the available information is from a patient series provided by the manufacturer that included 241 implant procedures carried out in 10 centers (67% biventricular VADs), with an overall survival rate of 83% at 6 months and 81% at 1 year.5 These data could be assumed to reflect the results of selected centers with considerable experience, but the figures are similar to those of a single-center report published in 2013 including 54 patients receiving circulatory support with the same VAD. As in our series, 24% had biventricular support, and the overall survival at 13 months was 80%.6
To compare these outcomes with those of other types of support, the INTERMACS registry from the United States contains information on implantation of more than 12 000 long-term devices since 2008 (95% intracorporeal continuous-flow left VADs). Survival rates of 80% at 1 year and 70% at 2 years have been reported.7 The European EUROMACS registry contains data on more than 700 devices (> 90% continuous-flow VADs), with survival rates of 68% at 1 year and 59% at 2 years. In contrast to INTERMACS, around 20% of the VADs implanted in EUROMACS have been biventricular.8 Biventricular support is associated with poorer outcomes in both registries, with 1-year survival rates of 50% in INTERMACS and 26% in EUROMACS.
In Spain, the number of devices implanted is significantly lower than in neighboring countries. This may be because of the high cost of these devices, together with the relative ease of access to CTx within a reasonable waiting period in our setting.9
The experience reported here with the first 25 patients who underwent EXCOR implantation suggests that this strategy would be useful in the following situations:
- •
Patients on the elective CTx waiting list who have progressive clinical deterioration, loss of muscle mass, injury to other organs, repeat hospitalizations, or dependence on inotropic agents (INTERMACS 2 and 3). This was the reason for VAD implantation in 12 patients in the present study. These patients did not have criteria for urgent CTx; hence, they could have ultimately undergone transplantation in a poorer condition or after use of short-term support, with the consequent increase in risk.10
- •
Patients with a contraindication for CTx, such as refractory pulmonary hypertension, which could be reversible in the mid- term with the use of a VAD. Until recently, these patients were rejected for CTx or were transplanted assuming a high risk of RV failure following transplant.
- •
Patients with a high risk of RV failure following implantation of a left VAD. This complication worsens the prognosis of patients on circulatory support.11 One option for these patients is elective implantation of a biventricular VAD, a strategy that cannot be carried out with the current intracorporeal continuous-flow devices. This approach was effective for reaching CTx in 5 of 6 patients in the present series, although most of them experienced unfavorable outcomes following transplant, which were directly related to technical problems and surgical complications. These results contrast with those of another limited experience in Spain that did have positive outcomes.12 The basic problem is that currently there are no good options for long-term support in patients with severe biventricular dysfunction. The results are disappointing in all the published registries. In the light of these considerations, biventricular VAD implantation should be reserved for selected patients who have no better treatment options, such as implantation of a short-term biventricular assist device and urgent transplantation, or implantation of a left VAD with temporary pharmacological or mechanical RV support measures.
The type and frequency of complications occurring in patients with pulsatile-flow support in the present study were comparable to those described for other types of circulatory support; similarly, most occurred within the first 30 days following the procedure. In the ADVANCE study,13 which used the continuous-flow HeartWare device as BTT, 17% of patients experienced a device-related infection (20% in the present study), 27% had severe bleeding events (28% in the present study), and 20% experienced stroke, a percentage somewhat lower than in the present study, where 32% of patients had highly lethal stroke complications. Most strokes occurred in the first patients in the series and during the first days following the procedure; hence, we cannot exclude an influence of the learning curve. With regard to this complication, the potential advantage of the EXCOR over intracorporeal devices is that the ventricle is external and transparent. Thrombus formation in the interior can be visualized, allowing replacement of the component before embolization or malfunction of the VAD occurs, as was done in 4 patients.
As to costs, it is evident that the need to replace a ventricle in 7 of the 25 patients included increased the overall cost of this approach. Even so, it would be reasonable to assume that the cost with this type of support would be lower than with continuous-flow support because of the difference in price between the 2 devices (a pulsatile-flow VAD costs about one-third that of a continuous-flow device). Furthermore, although the need for replacement due to thrombosis or dysfunction is much less frequent in continuous-flow than in pulsatile-flow VADs, it does occur in a certain percentage (around 10% at 2 years according to INTERMACS data). However, the present study was not designed to evaluate costs related to the technique.
LimitationsOne of the limitations of this study is that the analysis was based on a retrospective, single-center registry (although data were collected in real time and the database was specifically designed and used since the first case), and it included a relatively small number of patients and encompassed the learning curve. This last factor was mitigated by the help of an experienced surgeon in the first cases, and by training sessions for the team in centers with experience in VAD procedures. Irrespective of these considerations, the series illustrates the possibilities that this modality of VAD use has in Spain in actual clinical practice.
CONCLUSIONSThe results of this series demonstrate that the strategy of long-term pulsatile VAD implantation of the EXCOR type for BTT in a country such as Spain (where waiting list times are not extremely lengthy) is feasible and provides survival rates to CTx similar to those obtained with continuous-flow intracorporeal devices. The incidence of complications is relatively high, but the events are usually manageable, with the exception of those of neurological origin.
CONFLICTS OF INTERESTS. Serrano Fiz is a proctor of Berlin Heart for the EXCOR ventricular assist device.
- –
Ventricular assist devices have undergone rapid development in recent years and have proven useful as BTT and definitive treatment in patients with advanced HF.
- –
Most devices in current use are continuous-flow intracorporeal pumps, whose limitations include their high cost and difficulty in providing biventricular support.
- –
The number of ventricular assist devices implanted in Spain is significantly lower than in the neighboring countries.
- –
The main reason may be the relative ease of access to cardiac transplantation within a reasonable waiting time.
- –
This study describes the largest series of patients in Spain treated with a long-term pulsatile-flow VAD as cardiac BTT. The use of this type of circulatory support device is feasible in a country where the mean time on the transplant waiting list is relatively short and the economic situation is difficult.
- –
The possibility of providing biventricular support favors the use of these devices.
- –
There is a high percentage of associated complications, but they are usually manageable and comparable to those described for continuous-flow intracorporeal devices.
This study could not have been carried out without the collaboration of the entire staff of the Cardiology, Cardiac Surgery, Anesthesia, and Reanimation Departments of the Puerta de Hierro University Hospital of Majadahonda. The authors express special thanks to Drs Dolores García-Cosío, Inés Sayago and Natalia Jaramillo, to Gustavo Blaires for data processing support, and to Ana Royuela for help with the statistical analyses.