Individuals with a decreased estimated glomerular filtration rate (eGFR) are at increased risk of all-cause (ACM) and cardiovascular mortality; there is ongoing debate about whether older individuals with eGFR 45 to 59mL/min/1.73 m2 are also at increased risk. We evaluated the association between eGFR and ACM and cardiovascular events (CVE) in people aged 60 to 74 and ≥ 75 years in a population with a low coronary disease incidence.
MethodsWe conducted a retrospective cohort study by using primary care and hospital electronic records. We included 130 233 individuals aged ≥ 60 years with creatinine measurement between January 1, 2010 and December 31, 2011; eGFR was estimated by using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. The independent association between eGFR and the risk of ACM and hospital admission due to CVE were determined with Cox and Fine-Gray regressions, respectively.
ResultsThe median was age 70 years, and 56.1% were women; 13.5% had eGFR < 60 (69.7% eGFR 45-59). During a median follow-up of 38.2 months, 6474 participants died and 3746 had a CVE. For ACM and CVE, the HR in older individuals became significant at eGFR < 60. Fully adjusted HR for ACM in the eGFR 45 to 59 category were 1.61; 95%CI, 1.37-1.89 and 1.19; 95%CI, 1.10-1.28 in 60- to 74-year-olds and ≥ 75-year-olds, respectively; for CVE HR were 1.28; 95%CI, 1.08-1.51 and 1.12; 95%CI, 0.99-1.26.
ConclusionsIn a region with low coronary disease incidence, the risk of death and CVE increased with decreasing eGFR. In ≥ 75-year-olds, the eGFR 45 to 59 category, which had borderline risk for CVE, included many individuals without significant additional risk.
Keywords
An estimated glomerular filtration rate (eGFR) lower than 60mL/min/1.73 m2 has been found to be associated with increased risk of all-cause and cardiovascular mortality in general and high-risk populations.1,2 Individuals with decreased eGFR are more likely to die from cardiovascular causes than from kidney failure.1,3 The prevalence of eGFR < 60mL/min/1.73 m2 increases progressively with age, being as high as 50% in persons older than 80 years.4,5 Given population aging worldwide, renal function is expected to have a growing impact on cardiovascular disease in coming years.
Some studies have reported that the association between eGFR and mortality is attenuated by age.1,6 Moreover, there is ongoing debate about the clinical significance of moderately reduced kidney function (eGFR 45-59mL/min/1.73 m2) in people older than 65 years.7,8 Mortality was found to be significantly higher at eGFR < 60 in some studies,6,9 but only at eGFR < 45 in others.4,10–12 The results for cardiovascular events (CVE) are even more inconsistent.12,13 This scenario has led some authors to propose an age-calibrated threshold, ie, eGFR < 45 in people older than 65 years,8 which is interesting because of the high prevalence of milder reductions in older people.
Some of the discrepancies in the results may be due to differences in the methods used to measure creatinine, eGFR estimating equations, or the characteristics of the populations studied. Moreover, studies in areas with a low incidence of coronary heart disease (CHD) did not include individuals older than 74 years14,15 or did not provide detailed information across age groups.16,17
The objective of this study was to evaluate the age-specific association between eGFR and all-cause mortality (ACM) and the incidence of cardiovascular disease in individuals older than 60 years in a southern Europe population with a low incidence of CHD.18
MethodsDesign and Study PopulationWe performed a retrospective cohort study including all individuals born in or before 1950 who were registered in one of 40 primary health care centers with a centralized laboratory forming part of the Costa de Ponent Primary Care Service in north-east Spain (serving a population of 873 549 individuals), and whose creatinine was measured between January 1, 2010 and December 31, 2011. We excluded patients with kidney disease stage 5 (eGFR < 15, kidney transplant, or dialysis), those receiving health care at home, and those with < 30 days’ follow-up.
Data SourcesBaseline clinical data were obtained from primary care electronic medical records; for baseline cardiovascular disease, we also accounted for prior hospital admission due to cardiovascular disease or revascularization procedures since 2005. We defined the index date as that when the first creatinine measurement was taken during the inclusion period; baseline status was defined according to the characteristics registered between 1 year before and 1 month after the index date.
Renal Function AssessmentSerum creatinine levels were measured by a single laboratory using the standardized Jaffe compensated kinetic method traceable to an isotope dilution mass spectrophotometry reference method. The eGFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation19 without correction for race, which was not available.
We classified eGFR into clinical categories according to the KDIGO 2012 Clinical Practice Guideline: 15 to 29, 30 to 44, 45 to 59, 60 to 89, and 90 to 119.20
CovariatesWe collected data on age, sex, smoking status (never smoker, active smoker, and former smoker), hypercholesterolemia (serum cholesterol > 6.4 mmol/L or statin treatment), cardiovascular risk factors (hypertension, diabetes mellitus), and previous cardiovascular disease diagnosis (CHD, cerebrovascular disease, peripheral artery disease, and heart failure).
OutcomesData on date of death were obtained from hospital or administrative registers without cause specification; all endpoints for CVE were obtained from hospitalization records, which include data from all hospitals in Catalonia, the north-eastern region of Spain. The primary endpoint was ACM and the secondary endpoint was any CVE, including CHD (acute myocardial infarction [ICD-9—International Classification of Diseases, Ninth Revision—codes: 410, 412], unstable angina [411] or angina [413]) and stroke (nonhemorrhagic stroke [ICD-9 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, 434.91], or transient ischemic attack [435]). We analyzed hard CVE (acute myocardial infarction and nonhemorrhagic stroke), CHD, and stroke separately.
Participants were followed up from 1 month after the index date until they died, moved to another health system, or until the end of the study (December 31, 2013).
The study protocol was approved by the local clinical research ethics committee (IDIAP [Institut Universitari d’Investigació en Atenció Primària] Jordi Gol P11/43). We did not seek informed consent from participants, as it was not deemed necessary by the research ethics committee.
Statistical AnalysisAll variables were analyzed by age group (60 to 74 years and ≥ 75 years). We used these age groups because they coincide with most current systems for estimating risk of cardiovascular disease.18 Continuous variables are described as mean ± standard deviation or median [interquartile range] (nonnormally distributed), and categorical variables are described as absolute and relative frequencies. We used the Student t test and Kruskall-Wallis test to evaluate differences between groups of normally and nonnormally distributed continuous variables, respectively. We used the chi-square test for categorical variables, and also tested for linear trend between eGFR categories. Incident events were calculated using Kaplan-Meier curves at 3 years.
Within each group, we used Cox proportional hazards models to estimate hazard ratios (HRs) for the association between eGFR as a continuous variable and the risk of ACM. We verified the linearity of the eGFR effect using linear splines with 4 degrees of freedom in the Cox models. Due to the small number of individuals with eGFR ≥ 120 (n = 14) and the diminished precision of estimates over this point, these individuals were excluded from further analyses. We also used Cox proportional hazards models to test for the association between categorical eGFR, defined by KDIGO 2012 clinical cutoff points, and ACM. For all CVE, analysis was performed using competing risks survival techniques (Fine-Gray proportional subdistributional hazards regression) to account for the possible effect of incident mortality on the estimates.
The assumption of proportionality of the hazard over time was assessed graphically (Schoenfeld residuals) for the exposure variable (eGFR).
Cox proportional hazards models for both continuous and categorical eGFR were adjusted for age, sex, smoking status, hypercholesterolemia, diabetes mellitus, hypertension, and previous cardiovascular disease (ischemic heart, cerebrovascular, peripheral artery disease or heart failure). The models were further adjusted for treatments with available data (statins and renin-angiotensin system drugs).
Although CKD-EPI was developed using a sample with a broad age range (18-97 years), few individuals aged 80 years and older were present. Therefore, we performed a sensitivity analysis using the Berlin Initiative Study-1 Equation,21 which was specifically developed in a community-based sample of individuals aged ≥ 70 years.
The final models were validated using the Hosmer-Lemeshow test and the area under the receiver operating characteristic curve. For both methods, censoring and competing events were taken into account.
All statistical analyses were performed using R version 3.2.3 (R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria), with 2-sided tests and P < .05.
RESULTSOf 138 040 eligible individuals (73.1% of the entire population aged ≥ 60 years in this area), 6797 were excluded because they were in home care, 509 had stage 5 kidney disease, and 501 had been followed up for < 30 days; thus, the final study population comprised 130 233 (Figure 1 of the supplementary material). There were no clinically significant differences between included and excluded individuals in terms of age (P = .374) or sex (56.1% and 55.1% women, respectively; P < .001).
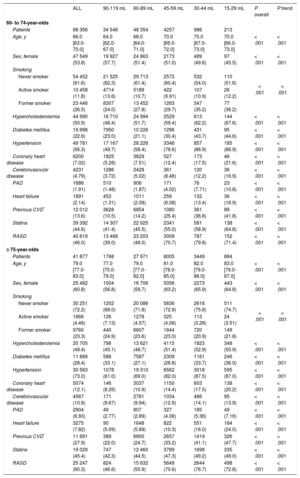
The median age of the entire sample was 70 years [64-76], and 56.1% of participants were women. The median eGFR was 82.42 [69.25-90.87]; 13.5% of participants had an eGFR < 60, of which 69.7% had eGFR of 45 to 59. When the sample was stratified by age (Table 1), the prevalence of eGFR < 60 was 6.18% in 60- to 74-year-olds and 29.0% in ≥ 75-year-olds (P < .001). The younger group had a lower proportion of women, diabetes mellitus, hypertension, and previous cardiovascular disease, and a higher proportion of active smokers (P < .001). Within each age group, comorbidity increased when eGFR was lower than the 60 to 89mL/min/1.73 m2 category.
Baseline Characteristics of the Study Population, Stratified by Age and Estimated Glomerular Filtration Rate Category
| ALL | 90-119 mL | 60-89 mL | 45-59 mL | 30-44 mL | 15-29 mL | P overall | P trend | |
|---|---|---|---|---|---|---|---|---|
| 60- to 74-year-olds | ||||||||
| Patients | 88 356 | 34 546 | 48 354 | 4257 | 986 | 213 | ||
| Age, y | 66.0 [63.0-70.0] | 64.0 [62.0-67.0] | 68.0 [64.0-71.0] | 70.0 [66.0-72.0] | 70.0 [67.0-73.0] | 70.0 [66.0-73.0] | < .001 | < .001 |
| Sex, female | 47 549 (53.8) | 19 927 (57.7) | 24 863 (51.4) | 2173 (51.0) | 489 (49.6) | 97 (45.5) | < .001 | < .001 |
| Smoking | < .001 | < .001 | ||||||
| Never smoker | 54 452 (61.6) | 21 525 (62.3) | 29 713 (61.4) | 2572 (60.4) | 532 (54.0) | 110 (51.6) | ||
| Active smoker | 10 458 (11.8) | 4714 (13.6) | 5189 (10.7) | 422 (9.91) | 107 (10.9) | 26 (12.2) | ||
| Former smoker | 23 446 (26.5) | 8307 (24.0) | 13 452 (27.8) | 1263 (29.7) | 347 (35.2) | 77 (36.2) | ||
| Hypercholesterolemia | 44 990 (50.9) | 16 710 (48.4) | 24 994 (51.7) | 2529 (59.4) | 613 (62.2) | 144 (67.6) | < .001 | < .001 |
| Diabetes mellitus | 19 998 (22.6) | 7950 (23.0) | 10 226 (21.1) | 1296 (30.4) | 431 (43.7) | 95 (44.6) | < .001 | < .001 |
| Hypertension | 49 781 (56.3) | 17 167 (49.7) | 28 226 (58.4) | 3346 (78.6) | 857 (86.9) | 185 (86.9) | < .001 | < .001 |
| Coronary heart disease | 6200 (7.02) | 1825 (5.28) | 3629 (7.51) | 527 (12.4) | 173 (17.5) | 46 (21.6) | < .001 | < .001 |
| Cerebrovascular disease | 4231 (4.79) | 1286 (3.72) | 2428 (5.02) | 361 (8.48) | 120 (12.2) | 36 (16.9) | < .001 | < .001 |
| PAD | 1686 (1.91) | 510 (1.48) | 906 (1.87) | 171 (4.02) | 76 (7.71) | 23 (10.8) | < .001 | < .001 |
| Heart failure | 1891 (2.14) | 453 (1.31) | 1011 (2.09) | 259 (6.08) | 132 (13.4) | 36 (16.9) | < .001 | < .001 |
| Previous CVD* | 12 012 (13.6) | 3628 (10.5) | 6854 (14.2) | 1080 (25.4) | 361 (36.6) | 89 (41.8) | < .001 | < .001 |
| Statins | 39 392 (44.6) | 14 307 (41.4) | 22 025 (45.5) | 2341 (55.0) | 581 (58.9) | 138 (64.8) | < .001 | < .001 |
| RASD | 40 619 (46.0) | 13 468 (39.0) | 23 203 (48.0) | 3009 (70.7) | 787 (79.8) | 152 (71.4) | < .001 | < .001 |
| ≥ 75-year-olds | ||||||||
| Patients | 41 877 | 1768 | 27 971 | 8005 | 3449 | 684 | ||
| Age, y | 79.0 [77.0-83.0] | 77.0 [75.0-79.0] | 79.0 [77.0-82.0] | 81.0 [78.0-85.0] | 82.0 [79.0-86.0] | 83.0 [79.0-87.0] | < .001 | < .001 |
| Sex, female | 25 482 (60.8) | 1004 (56.8) | 16 706 (59.7) | 5056 (63.2) | 2273 (65.9) | 443 (64.8) | < .001 | < .001 |
| Smoking | < .001 | < .001 | ||||||
| Never smoker | 30 251 (72.2) | 1202 (68.0) | 20 086 (71.8) | 5836 (72.9) | 2616 (75.8) | 511 (74.7) | ||
| Active smoker | 1866 (4.46) | 126 (7.13) | 1278 (4.57) | 325 (4.06) | 113 (3.28) | 24 (3.51) | ||
| Former smoker | 9760 (23.3) | 440 (24.9) | 6607 (23.6) | 1844 (23.0) | 720 (20.9) | 149 (21.8) | ||
| Hypercholesterolemia | 20 705 (49.4) | 798 (45.1) | 13 621 (48.7) | 4115 (51.4) | 1823 (52.9) | 348 (50.9) | < .001 | < .001 |
| Diabetes mellitus | 11 889 (28.4) | 586 (33.1) | 7587 (27.1) | 2309 (28.8) | 1161 (33.7) | 246 (36.0) | < .001 | < .001 |
| Hypertension | 30 563 (73.0) | 1078 (61.0) | 19 310 (69.0) | 6562 (82.0) | 3018 (87.5) | 595 (87.0) | < .001 | < .001 |
| Coronary heart disease | 5074 (12.1) | 146 (8.26) | 3037 (10.9) | 1150 (14.4) | 603 (17.5) | 138 (20.2) | < .001 | < .001 |
| Cerebrovascular disease | 4567 (10.9) | 171 (9.67) | 2781 (9.94) | 1034 (12.9) | 486 (14.1) | 95 (13.9) | < .001 | < .001 |
| PAD | 2904 (6.93) | 49 (2.77) | 807 (2.89) | 327 (4.08) | 185 (5.36) | 49 (7.16) | < .001 | < .001 |
| Heart failure | 3275 (7.82) | 90 (5.09) | 1648 (5.89) | 822 (10.3) | 551 (16.0) | 164 (24.0) | < .001 | < .001 |
| Previous CVD* | 11 691 (27.9) | 389 (22.0) | 6900 (24.7) | 2657 (33.2) | 1419 (41.1) | 326 (47.7) | < .001 | < .001 |
| Statins | 19 029 (45.4) | 747 (42.3) | 12 460 (44.5) | 3789 (47.3) | 1698 (49.2) | 335 (49.0) | < .001 | < .001 |
| RASD | 25 247 (60.3) | 824 (46.6) | 15 632 (55.9) | 5649 (70.6) | 2644 (76.7) | 498 (72.8) | < .001 | < .001 |
CVD, cardiovascular disease; PAD, peripheral artery disease; RASD, renin-angiotensin system drugs.
Unless otherwise indicated, data are expressed as No. (%) or median [interquartile range].
To test for eGFR associations in the Cox models, we defined eGFR = 80 as the reference (also used in the CKD-Prognostic Consortium[CKD-PC] meta-analysis)6 in both groups because this was the median eGFR in the population, and 60 to 90 as the clinical category, as this included the highest number of individuals and was clinically significant.
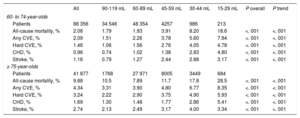
The median follow-up was 38.2 months [37.2-42.7]. A total of 6474 deaths, 1573 CHD events, and 2236 cerebrovascular events were recorded. All outcomes were significantly more common in the older age group (P < .001), and in each successively lower eGFR category (Table 2), except for stroke events, which were slightly less common in individuals with an eGFR of 15 to 29.
Outcomes in the Study Population, Stratified by Age and Estimated Glomerular Filtration Rate According to Kaplan-Meier Estimates at 3 Years. Log Rank Test (P Overall), Linear Trend Wald Test (P Trend)
| All | 90-119 mL | 60-89 mL | 45-59 mL | 30-44 mL | 15-29 mL | P overall | P trend | |
|---|---|---|---|---|---|---|---|---|
| 60- to 74-year-olds | ||||||||
| Patients | 88 356 | 34 546 | 48 354 | 4257 | 986 | 213 | ||
| All-cause mortality, % | 2.08 | 1.79 | 1.93 | 3.91 | 8.20 | 18.6 | <. 001 | <. 001 |
| Any CVE, % | 2.09 | 1.51 | 2.26 | 3.78 | 5.60 | 7.94 | <. 001 | <. 001 |
| Hard CVE, % | 1.46 | 1.06 | 1.56 | 2.76 | 4.05 | 4.78 | <. 001 | <. 001 |
| CHD, % | 0.96 | 0.74 | 1.02 | 1.38 | 2.83 | 4.80 | <. 001 | <. 001 |
| Stroke, % | 1.16 | 0.79 | 1.27 | 2.44 | 2.88 | 3.17 | <. 001 | <. 001 |
| ≥ 75-year-olds | ||||||||
| Patients | 41 877 | 1768 | 27 971 | 8005 | 3449 | 684 | ||
| All-cause mortality, % | 9.88 | 10.5 | 7.89 | 11.7 | 17.8 | 28.5 | <. 001 | <. 001 |
| Any CVE, % | 4.34 | 3.31 | 3.90 | 4.80 | 6.77 | 8.35 | <. 001 | <. 001 |
| Hard CVE, % | 3.24 | 2.22 | 2.90 | 3.75 | 4.90 | 5.93 | <. 001 | <. 001 |
| CHD, % | 1.69 | 1.30 | 1.48 | 1.77 | 2.86 | 5.41 | <. 001 | <. 001 |
| Stroke, % | 2.74 | 2.13 | 2.49 | 3.17 | 4.00 | 3.34 | <. 001 | <. 001 |
CHD, coronary heart disease; CVE, cardiovascular event.
Both models, for ACM and any CVE, showed proportionality of the hazard over time for the exposure variable (eGFR; data not shown).
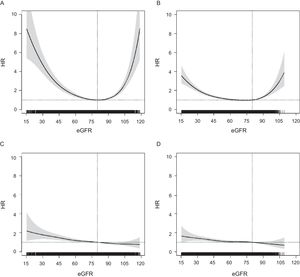
The association between eGFR and risk of ACM followed a U-shaped pattern in both age groups but was more attenuated in the older group (Figure 1). In contrast, the HRs for any CVE showed a linear distribution, with a progressive increase in risk from higher eGFR to lower; this pattern was similar in both age groups, and also for other cardiovascular endpoints (Figure 2 of the supplementary material). For all outcomes, the HRs in ≥ 75-year-olds became significant below eGFR = 60 (Figure 1), between eGFR = 55 and eGFR = 60 for ACM and at eGFR ∼ 50 for any CVE.
Association between eGFR (continuous variable) and risk of ACM in persons aged < 75 years and ≥ 75 years-old (A and B) and risk of any cardiovascular event (C and D) assessed using adjusted Cox proportional hazards models considering death as a competing event for CVEs (overall P value < .001 for each model). Adjusted HR point estimation (95%CI) at eGFR = 60, eGFR = 55 and eGFR = 50 (vs eGFR = 80). A: (ACM in persons aged < 75 years): HR, 1.486; 95%CI, 1.315-1.680; HR, 1.757; 95%CI, 1.530-2.018 and HR, 2.100; 95%CI, 1.806-2.442, respectively. B: (ACM in persons aged ≥ 75 years): HR, 1.056; 95%CI, 0.978-1.139; HR, 1.129; 95%CI, 1.043-1.223 and HR, 1.226; 95%CI, 1.130-1.329, respectively. C: (any CVE in persons aged < 75 years): HR, 1.185; 95%CI, 1.039-1.350; HR, 1.263; 95%CI, 1.088-1.465 and HR, 1.365; 95%CI, 1.158-1.608, respectively. D: (any cardiovascular event in persons aged ≥ 75 years): HR, 1.053; 95%CI, 0.935-1.187; HR, 1.082; 95%CI, 0.955-1.226 and HR, 1.133; 95%CI, 0.996-1.288, respectively. 95%CI, 95% confidence interval; ACM, all-cause mortality; CVE, cardiovascular event; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
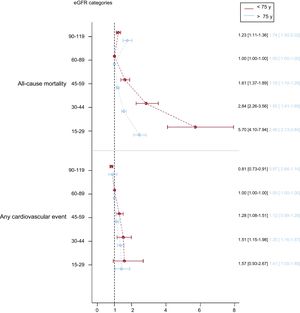
In terms of the clinical categories of eGFR, risk of ACM and any CVE gradually increased in eGFR categories below 60 to 89 in both age groups (Figure 2). The increase in risk of ACM was much higher and steeper in 60- to 74-year-olds than in ≥ 75-year-olds, whereas that for any CVE was more similar in both age groups and was borderline significant in the eGFR = 45 to 59 category for ≥ 75-year-olds. Further adjustment for treatment with statins and renin-angiotensin system drugs did not modify the HR values (data not shown). We also found that individuals with an eGFR of 90 to 119 had a higher risk of death in the oldest group and a significantly lower risk of any CVE in the youngest group only. The results of models for CHD, stroke, and hard CVE were similar to those for any CVE (Figure 3 of the supplementary material). A sensitivity analysis using the Berlin Initiative Study-1 Equation to calculate eGFR showed no differences (Figure 4 of the supplementary material).
Association between eGFR categories and risk of all-cause mortality and any cardiovascular event, assessed using adjusted Cox proportional hazards models considering death as a competing event for cardiovascular events (overall P value < .001 for each model). eGFR, estimated glomerular filtration rate.
The final models showed good calibration and discrimination (Figure 5 of the supplementary material and Table of the supplementary material).
DISCUSSIONIn a population of individuals aged ≥ 60 years in a country with low cardiovascular risk, the risk of ACM and any CVE increased gradually with decreasing eGFR, both in 60- to 74-year-olds and in ≥ 75-year-olds, independently of other risk factors and cardiovascular disease. However, the HRs in the older age group became significant at lower eGFR (below eGFR = 60), and were borderline significant for any CVE in the eGFR = 45 to 59 category. The eGFR-associated increase in mortality risk was higher in 60- to 74-year-olds than in ≥ 75-year-olds, but was similar for CVE risk. Moreover, the association between eGFR and mortality followed a U-shaped pattern, with increased mortality in the 90 to 119 category and was higher in ≥ 75-year-olds.
In this study population, the age categories defined 2 well-differentiated groups, in which the number of individuals with eGFR < 60 in ≥ 75-year-olds was 5-fold that in 60- to 74-year-olds. All cardiovascular risk factors and outcomes were also more common in the older group, with approximately 5-fold higher ACM and 2-fold higher risk of CVE.
As expected, the incidence of cardiovascular outcomes was lower than reported in elderly individuals in non-Mediterranean countries,22 but was higher than in younger individuals in our area.23 Incident cerebrovascular events were more common than coronary events, which has also been described in studies of elderly people.22
Consistent with previous reports,1,6,14,22,24 we observed a U-shaped relationship between eGFR as a continuous variable and ACM in both age groups. Remarkably, the excess risk of ACM observed in the eGFR = 90 to 119 category was higher in older patients. This increase in risk could reflect confounding by muscle wasting secondary to other illnesses leading to death; this is a known limitation of creatinine-based estimating formulas.19 In contrast, we found that risk of new CVE increased from higher to lower eGFR. This is consistent with some previous reports,13,23,25 although others have reported a less marked U-shape relationship for cardiovascular mortality6,12 and cardiovascular disease.15 Thus, incident CVE, which appear to be less affected by the limitations of creatinine-based estimating formulas, could be a more reliable index of the prognostic effect of eGFR on cardiovascular risk.
There is conflicting evidence on the clinical significance of moderately reduced eGFR in older people. Few studies have used the currently recommended equation to assess eGFR-associated risk and to compare results across age categories. To our knowledge, ours is the first study to do this in an older population residing in a region with a low incidence of CHD.
For ACM, the CKD-PC meta-analysis6 reported a significant adjusted HR at eGFR < 60 in 65 to 74 year-olds and ≥ 75 year-olds, and the REGARDS study9 made a similar finding in 60 to 69, 70 to 79, and ≥ 80 year-olds. In contrast, in octogenarians, the Cardiovascular Health Study12 only found significant HRs at eGFR ≤ 43. In southern Europe, mortality has been found to increase in persons older than 65 years with eGFR < 45,16 and in 60 to 74 year-olds with eGFR < 90.15 In 35 to 74 year-olds without cardiovascular disease, eGFR was found to be a borderline significant predictor of ACM,14 and with significantly greater risk at eGFR = 45-59.
For cardiovascular mortality, the HRs in the groups aged 65 to 74 and ≥ 75 years were significant for eGFR < 60 in the CKD-PC meta-analysis,6 whereas the Cardiovascular Health Study, using creatinine-based equations, did not find a similar association in octogenarians.12 Regarding the risk of incident CVE, the PREVEND study found an association with eGFR in persons younger than 60 years, but not in those aged ≥ 60 years.13 In southern Europe, an increased risk of cardiovascular mortality has been reported at eGFR < 60 in persons older than 65 years,16 and of incident cardiovascular disease (marginally significant) at eGFR < 90 in 60- to 74-year-olds,15 and eGFR < 60 in 35- to 74-year-olds.23
In our study, the HRs for ACM and any CVE increased steadily with decreasing eGFR below the 60 to 89 category, in both the groups aged 60 to 74 and ≥ 75 years. As in previous reports, the HR values and risk gradient were lower in the oldest group,6,9 and on analysis of continuous eGFR, HRs became significant at eGFR < 60.6
Most current international guidelines consider all individuals with eGFR < 60 to have a high risk of CVE, an established CHD risk equivalent, and recommend aggressive management accordingly.18,20,26 In the present study, 20% of participants aged ≥ 75 years had eGFR = 45 to 59, of whom 74% had eGFR ≥ 50. Thus, considering all older individuals in the 45 to 59 category as having higher risk would expose many individuals with ‘no significant added risk’ to more aggressive targets and treatment for cardiovascular risk factors. Additionally, the 11% increased relative risk of CHD events in 60- to 74-year-olds might not be sufficient to consider the eGFR = 45 to 59 category as a CHD risk equivalent in countries with a low incidence of CHD.
Strengths and LimitationsA major strength of our study is the very large number of individuals included, and the fact that they were drawn from a representative population-based sample, reflecting the true situation of patients managed in primary care. Nearly all individuals aged > 60 years (93%) were attended by the Catalan Primary Health Care System, and a high percentage had a creatinine measurement, probably because this is part of the basic preventive health check-up and routine follow-up of other prevalent chronic conditions in this population. In addition, creatinine measurement assays were calibrated to a reference method using isotope dilution mass spectrophotometry as recommended, and were performed in a centralized laboratory, which further reduces variability.
The results of this study should also be interpreted in light of various limitations. First, eGFR values and categories were assigned on the basis of a single measurement, which is usual in epidemiological studies of prognosis. Because of the regression dilution bias, this can underestimate the true association between eGFR and the outcomes of interest. However, low eGFR measurements from ambulatory laboratory databases have been shown to be relatively stable in older individuals.27 Second, we did not account for the effect of some factors that can modify renal function such as neoplasms, nephrotoxic drugs, infections, or clinically intercurrent events. We believe these effects are minimized by the large number of patients included. Third, we estimated eGFR from serum creatinine measurements using the CKD-EPI formula, and we accept the limitations of creatinine-based estimating formulas, which have greater impact at higher eGFR.19 We did not correct for race, although Caucasian ethnicity is predominant in our population, especially in this age range. Furthermore, while the diagnostic and prognostic use of CKD-EPI in older individuals is subject to debate and new formulas have been developed, CKD-EPI is currently the recommended approach20 and is widely used in primary care. Moreover, a sensitivity analysis with eGFR calculated according to the Berlin Initiative Study-1 Equation yielded similar results. Fourth, we were unable to evaluate the effect of urine albumin, as these data were not available; while eGFR and albumin are both recommended for evaluating CKD, they have shown independent prognostic implications,1 and the results presented here add valuable information about the effect of eGFR on cardiovascular risk and cutoff levels in older individuals. Fifth, data were obtained from electronic health records, and misdetection cannot be excluded. Data for cardiovascular disease in primary health care has been shown to be of higher quality than for other diseases and suitable for epidemiological studies in our population.28,29 Fatal CVE outside the hospital were not included. Specifically, we may have underdetected stroke events in patients with advanced kidney disease who were not admitted to the hospital, and these were likely more severe and with greater comorbidity resulting in death. The pattern for stroke events in individuals with eGFR between 45 and 80 was similar to that for other CVE; we do not believe that this invalidates our results, since our focus was on mild CKD. Sixth, models were adjusted for cardiovascular risk factors and diseases, as well as statin therapy and renin-angiotensin-system drugs, but not for other comorbidities, drugs or socioeconomic factors that may affect the incidence of CVE. Finally, we did not have access to data on cause of death. All-cause mortality includes several etiologies that are not related to renal function but to other age-associated diseases, diabetes mellitus or heart failure, such as infections or falls. While we adjusted for some of these comorbidities, we cannot rule out the presence of other sources of confounding, such as frailty.30
CONCLUSIONSIn conclusion, in people aged ≥ 60 years residing in a country with a low incidence of CHD, we observed an increase in risk of ACM and any CVE with decreasing eGFR in both the groups aged 60 to 74 years and ≥ 75 years. However, HRs became significant at eGFR < 60 in the older group and were borderline significant in the eGFR = 45 to 59 category for any CVE. Our results suggest that individuals aged ≥ 75 years in the most common category, that of mildly to moderately decreased eGFR = 45 to 59, should not be considered to have increased overall risk, as this group includes many individuals without significant additional risk. Moreover, the increased risk in the eGFR = 45 to 59 category in 60- to 74-year-olds may not be enough to be considered a coronary risk equivalent in regions with a low incidence of CHD.
FUNDINGThis project was supported by a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded under the 2011 call of the Health Strategy Action, within the National Research Program oriented to Societal Challenges. This program forms part of the Technical, Scientific and Innovation Research National Plan 2008-2011, cofunded with European Union ERDF (European Regional Development Fund) funds (PI11/02220). Ministry of Economy and Competitiveness through the Carlos III Institute of Health (Red RedIAPP RD12/0007) and ERDF funds Generalitat de Catalunya through the AGAUR (A for Management of Universities and Research Grants) (2014 SGR 1225) (2014 SGR 902). M. Grau was funded by a Miguel Servet Grant (Carlos III Institute of Health, Ministry of Economy and Competitiveness, Spain) (PI12/03287).
CONFLICTS OF INTERESTL. Pascual-Benito received lecture fees from Alter. A. Martínez-Castelao received consulting fees/paid advisory boards from Boëhringer-Ingelheim, and lecture fees from and Boëhringer-Ingelheim.
- –
Individuals with an eGFR < 60mL/min/1.73 m2 are at increased cardiovascular risk. The prevalence of decreased eGFR increases progressively with age, being as high as 50% in persons older than 80 years. There is ongoing debate about the clinical significance of the more frequent milder reductions (eGFR 45 to 59mL/min/1.73 m2) in older people. Moreover, studies in areas with a low incidence of CHD have not included individuals older than 74 years or have not provided detailed information across age groups.
- –
In a population of individuals aged ≥ 60 years residing in a country with a low cardiovascular risk, the risk of ACM and any CVE increased gradually with decreasing eGFR, both in 60- to 74-year-olds and in ≥ 75-year-olds, independently of other risk factors and cardiovascular diseases. However, the HRs in the older age group became significant at eGFR below 60. The eGFR-associated increase in mortality risk was higher in the group aged 60 to 74 years than in the group aged ≥ 75 years but was similar for CVE risk.
We thank Dr Gavin Lucas and collaborators at ThePaperMill for their help with critical and linguistic review of the final version of the manuscript.