Long-term data on the relationship between endothelial dysfunction after ST-segment elevation myocardial infarction and future adverse clinical events are scarce. The aim of this study was to noninvasively assess whether endothelial dysfunction 4 weeks to 6 weeks after primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction predicts future clinical events.
MethodsThis prospective cohort study was performed in 70 patients of the RESPONSE randomized trial, who underwent noninvasive assessment of endothelial function 4 weeks to 6 weeks after primary percutaneous coronary intervention. Endothelial function was measured by the reactive hyperemia peripheral artery tonometry method; an index<1.67 identified endothelial dysfunction.
ResultsThe reactive hyperemia peripheral artery tonometry index measured on average 1.90±0.58. A total of 35 (50%) patients had endothelial dysfunction and 35 (50%) patients had normal endothelial function. Periprocedural “complications” (eg, cardiogenic shock, total atrioventricular block) were more common in patients with endothelial dysfunction than in those without (25.7% vs 2.9%; P<.01). During 4.0±1.7 years of follow-up, 20 (28.6%) patients had major adverse cardiovascular events: events occurred in 9 (25.7%) patients with endothelial dysfunction and in 11 (31.5%) patients with normal endothelial function (P=.52). There was an association between the prevalence of diabetes mellitus at baseline and the occurrence of major adverse cardiovascular events during follow-up (univariate analysis: hazard ratio=2.8; 95% confidence interval, 1.0-7.8; P<.05), and even in multivariate analyses the risk appeared to be increased, although not significantly (multivariate analysis: hazard ratio=2.5; 95% confidence interval, 0.8-7.5).
ConclusionsIn this series of patients who survived an ST-segment elevation myocardial infarction, endothelial dysfunction, as assessed by reactive hyperemia peripheral artery tonometry 4 weeks to 6 weeks after myocardial infarction, did not predict future clinical events during a mean follow-up of 4 years.
Keywords
Endothelial dysfunction of coronary conductance and resistance vessels, which is more often observed in the presence of certain cardiovascular risk factors, contributes significantly to the process of artherogenesis1–5 and may cause myocardial ischemia.6–8 The improvement of clinical outcome in response to modification of risk factors may primarily be the result of functional recovery of the impaired coronary vasomotor function, while structural changes of the atherosclerotic vessel wall remain largely unchanged.9 Previous studies have shown that endothelial dysfunction is associated with an increased event risk in patients without obstructive coronary lesions10–14 and with an increased restenosis risk after stent implantation in patients without myocardial infarction.15,16 During an average follow-up of 14 months, a population of patients with uncomplicated myocardial infarctions showed an increased event risk if they had both diabetes mellitus and endothelial dysfunction.17
However, so far, long-term data on clinical outcome in relation to endothelial function after ST-segment elevation myocardial infarction (STEMI) are scarce.18 Most of the aforementioned studies used noninvasive techniques to assess peripheral endothelial function, which has been shown to correlate with coronary endothelial function.19,20 While ultrasound-based methods for the assessment of flow-mediated dilatation of the brachial artery require significant training and experience, reactive hyperemia peripheral artery tonometry (RH-PAT) is an operator-independent method that has been validated against the ultrasound-based approach and with acetylcholine-based assessment of coronary endothelial function.21–23 In addition, an RH-PAT index of peripheral endothelial function has been shown to be reduced in the presence of proven coronary endothelial dysfunction.23
In the present prospective substudy of the RESPONSE trial,24 STEMI patients were examined with RH-PAT 4 weeks to 6 weeks after treatment by primary percutaneous coronary intervention (PPCI) in order to evaluate the hypothesis that endothelial dysfunction, as measured 4 weeks to 6 weeks from PPCI, may predict long-term clinical outcome after STEMI.
METHODSStudy Population and DesignThis prospective cohort study was performed in 70 STEMI patients of the RESPONSE trial,24 who underwent treatment by PPCI for acute STEMI (≤ 12hours after symptom onset) and noninvasive assessment of endothelial function with the RH-PAT method after 4 weeks to 6 weeks. The PPCI were performed between October 2007 and December 2008 at Thoraxcentrum Twente. Of a total of 75 STEMI patients of the RESPONSE trial with RH-PAT measurements, 71 had analyzable RH-PAT registrations, and follow-up was available in all but 1 of these 71 patients, resulting in the present study population of 70 patients. Patients were followed-up until a first major adverse cardiovascular event (MACE) occurred or, if event-free, until the end of the study in May 2013. From study enrollment until the end of follow-up, the following adverse events were documented: target vessel revascularization, coronary artery bypass grafting, interventions for obstructive peripheral artery disease, stroke, and death from any cause. The randomized RESPONSE trial quantified the impact of a practical, hospital-based, nurse-coordinated prevention program on cardiovascular risk, integrated into the routine clinical care of patients who were discharged after an acute coronary syndrome, as compared with usual care only. Risk factor control was then classified, based on the number of risk factors that were on target.24 In brief, patients had to be aged 18 years to 80 years, without surgery or additional PCI being planned within 8 weeks from PPCI, without congestive heart failure New York Heart Association functional class III or IV, and with a life-expectancy of at least 2 years.
As inflammation and repair processes of the infarcted myocardium might have disturbed endothelial function measurements during the first weeks after the STEMI and endothelial dysfunction would not have fully recovered under medication, endothelial function was assessed 4 weeks to 6 weeks after the PPCI.25–27 All patients were seen in the outpatient clinic and the research department of Thoraxcentrum Twente, where endothelial function was noninvasively assessed according to strict rules in a dedicated laboratory.28 There was no routine angiographic follow-up assessment. All patients provided written, informed consent for both participation in the RESPONSE trial and in the substudy. The trial and substudy complied with the Declaration of Helsinki for investigation in humans and were approved by the Medical Ethics Committee of Twente in Enschede, The Netherlands.
Coronary Intervention and Concomitant Medical TherapyPatients were treated in the ambulance with an intravenous bolus of 5000 IU of unfractionated heparin, a loading dose of ≥ 300mg of acetylsalicylic acid (orally or intravenously), and an oral loading dose of 600mg of clopidogrel. In 50 (71.4%) patients, a weight-adjusted intracoronary bolus of abciximab was administered after visualizing the culprit coronary artery. Primary PCI procedures were generally performed via the femoral route through 6 Fr sheaths; drug therapy, use of aspiration catheters, lesion preparation (vs direct stenting), and stent postdilatation were performed according to current guidelines and the operator's judgment and discretion. Following PPCI, a heart team carefully assessed the coronary angiographies and, if required, patients underwent a staged PCI for additional coronary lesions, which was generally performed within 1 week to 2 weeks from PPCI.
Noninvasive Assessment of Endothelial Function With the Reactive Hyperemia Peripheral Artery Tonometry MethodEndothelial function was evaluated with the RH-PAT method. The finger pulse wave amplitude was assessed with the EndoPAT-2000 sensing device and finger plethysmographic probes (Itamar Medical; Caesarea, Israel), both at baseline and during ischemia-induced hyperemia. All measurements were performed in the early morning in a dedicated laboratory after the patients had fasted for at least 8hours. The patients also had to refrain from caffeine consumption, smoking, and vasoactive medications. At least 15min prior to testing, blood pressure was measured and a blood sample was drawn in the control arm. Before any measurement, the patients had an acclimatization period of 20min in a quiet room, lying in a hospital bed at an ambient temperature of 21°C to 23°C.
The RH-PAT method has previously been reported in detail.28,29 In brief, measurements were performed by the use of probes on the index fingers of both the study and control arm. Baseline measurements were recorded for 5mins prior to ischemia induction by inflating a blood pressure cuff on the upper arm of the study arm for 5min to suprasystolic pressures. This led to nitric oxide-release from functional endothelium and thus vasodilatation, which was recorded by the sensors in the finger cuff through beat-to-beat finger pulsed wave analysis.30 Following the release of the blood pressure cuff, the ratio of the pulse amplitude of the hyperemic finger and the baseline amplitude was calculated. Subsequently, that ratio was divided by the corresponding ratio, obtained in the control arm, to calculate the RH-PAT index (high values indicate good endothelial function).30 Recently, Hamburg et al31 demonstrated that the maximum hyperemic response can be expected 90s to 120s after cuff deflation. Therefore, in the present study, the reactive RH-PAT index was calculated as the ratio of the mean hyperemic pulsed wave analysis over a period of 30s, beginning at 90s after cuff deflation, divided by the baseline pulsed wave analysis (mean baseline measurements for 3.5min), and normalized to the concurrent measurements of the control arm. Endothelial function was divided into 2 groups: 1 with endothelial dysfunction (RH-PAT<1.67) and 1 with normal endothelial function (RH-PAT ≥ 1.67).29
Patient Characteristics and DefinitionsThe following information was documented: age, sex, body mass index, (kg/m2); arterial hypertension (blood pressure of>140/90mmHg or treatment with antihypertensive medication), history of smoking (previous or current smoker), history of previous myocardial infarction and/or coronary revascularization by means of PCI or bypass surgery, time from symptom onset to PPCI, presence of diabetes mellitus (patient history and/or treatment with insulin or oral antiglycemic agents), and history of hyperlipidemia or treatment with lipid-lowering drugs.
A composite endpoint consisting of any death, myocardial infarction, coronary revascularization, stroke, or revascularization for newly developed peripheral arterial disease was called MACE.
Statistical AnalysesData are presented as frequencies (%) or mean±standard deviation. We used the chi-square test for categorical variables and the Student t test for continuous variables to compare patients with normal endothelial function vs patients with endothelial dysfunction. Kaplan-Meier MACE event-free curves were drawn for both patient groups. Differences between the 2 curves were tested using the log rank test. Univariate and multivariate Cox regression analyses were performed to assess the effect of endothelial dysfunction on MACE and adjust for traditional cardiovascular risk factors (diabetes mellitus, hypertension, hypercholesterolemia, smoking). Hazard ratios were calculated with 95% confidence interval. A P-value<.05 was considered statistically significant. All analyses were performed with SPSS (version 16.0).
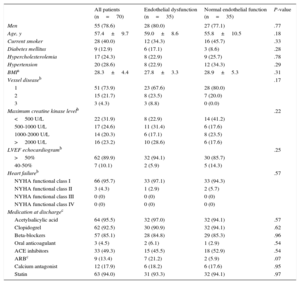
RESULTSPatient and Procedural Characteristics, in-hospital Course, and Endothelial FunctionOf all 70 patients, who had a mean age of 56.9±9.6 years, 55 (78.6%) were men. Primary PCI procedures were performed via the femoral route in all but one patient (98.4%); eight patients (11.4%) had prior percutaneous intervention and 1 (1.4%) had a history of coronary artery bypass grafting; manual thrombus aspiration was performed in 38 (62.3%), direct stenting in 36 (51.4%), and stent postdilatation in 56 (93.3%) patients. At discharge, the left venticular ejection fraction was preserved (> 50%) in 62 (89.9%) patients. Further information on the study population, interventional procedures, and in-hospital course are presented in Tables 1 and 2.
Demographics, Patient Characteristics, Parameters of Clinical Course and Left Ventricular Impairment, and Medication
| All patients (n=70) | Endothelial dysfunction (n=35) | Normal endothelial function (n=35) | P-value | |
|---|---|---|---|---|
| Men | 55 (78.6) | 28 (80.0) | 27 (77.1) | .77 |
| Age, y | 57.4±9.7 | 59.0±8.6 | 55.8±10.5 | .18 |
| Current smoker | 28 (40.0) | 12 (34.3) | 16 (45.7) | .33 |
| Diabetes mellitus | 9 (12.9) | 6 (17.1) | 3 (8.6) | .28 |
| Hypercholesterolemia | 17 (24.3) | 8 (22.9) | 9 (25.7) | .78 |
| Hypertension | 20 (28.6) | 8 (22.9) | 12 (34.3) | .29 |
| BMIa | 28.3±4.4 | 27.8±3.3 | 28.9±5.3 | .31 |
| Vessel diseaseb | .17 | |||
| 1 | 51 (73.9) | 23 (67.6) | 28 (80.0) | |
| 2 | 15 (21.7) | 8 (23.5) | 7 (20.0) | |
| 3 | 3 (4.3) | 3 (8.8) | 0 (0.0) | |
| Maximum creatine kinase levelb | .22 | |||
| <500 U/L | 22 (31.9) | 8 (22.9) | 14 (41.2) | |
| 500-1000 U/L | 17 (24.6) | 11 (31.4) | 6 (17.6) | |
| 1000-2000 U/L | 14 (20.3) | 6 (17.1) | 8 (23.5) | |
| >2000 U/L | 16 (23.2) | 10 (28.6) | 6 (17.6) | |
| LVEF echocardiogramb | .25 | |||
| >50% | 62 (89.9) | 32 (94.1) | 30 (85.7) | |
| 40-50% | 7 (10.1) | 2 (5.9) | 5 (14.3) | |
| Heart failureb | .57 | |||
| NYHA functional class I | 66 (95.7) | 33 (97.1) | 33 (94.3) | |
| NYHA functional class II | 3 (4.3) | 1 (2.9) | 2 (5.7) | |
| NYHA functional class III | 0 (0) | 0 (0) | 0 (0) | |
| NYHA functional class IV | 0 (0) | 0 (0) | 0 (0) | |
| Medication at dischargec | ||||
| Acetylsalicylic acid | 64 (95.5) | 32 (97.0) | 32 (94.1) | .57 |
| Clopidogrel | 62 (92.5) | 30 (90.9) | 32 (94.1) | .62 |
| Beta-blockers | 57 (85.1) | 28 (84.8) | 29 (85.3) | .96 |
| Oral anticoagulant | 3 (4.5) | 2 (6.1) | 1 (2.9) | .54 |
| ACE inhibitors | 33 (49.3) | 15 (45.5) | 18 (52.9) | .54 |
| ARBc | 9 (13.4) | 7 (21.2) | 2 (5.9) | .07 |
| Calcium antagonist | 12 (17.9) | 6 (18.2) | 6 (17.6) | .95 |
| Statin | 63 (94.0) | 31 (93.3) | 32 (94.1) | .97 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BMI, body mass index; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Data are expressed as No. (%) or mean ± standard deviation.
Percentages are calculated based on known values.
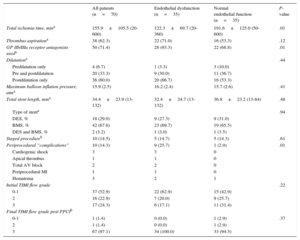
Details of Interventional Procedure and Coronary Angiographic Information
| All patients (n=70) | Endothelial dysfunction (n=35) | Normal endothelial function (n=35) | P-value | |
|---|---|---|---|---|
| Total ischemia time, mina | 155.9±105.5 (20-600) | 122.3±69.7 (20-360) | 191.6±125.0 (50-600) | .01 |
| Thrombus aspirationa | 38 (62.3) | 22 (71.0) | 16 (53.3) | .12 |
| GP IIb/IIIa receptor antagonists useda | 50 (71.4) | 28 (93.3) | 22 (68.8) | .01 |
| Dilatationa | .44 | |||
| Predilatation only | 4 (6.7) | 1 (3.3) | 3 (10.0) | |
| Pre and postdilatation | 20 (33.3) | 9 (30.0) | 11 (36.7) | |
| Postdilatation only | 36 (60.0) | 20 (66.7) | 16 (53.3) | |
| Maximum balloon inflation pressure, atma | 15.9 (2.5) | 16.2 (2.4) | 15.7 (2.6) | .41 |
| Total stent length, mma | 34.4±23.9 (13-132) | 32.4±24.7 (13-132) | 36.8±23.2 (13-84) | .48 |
| Type of stenta | .94 | |||
| DES, % | 18 (29.0) | 9 (27.3) | 9 (31.0) | |
| BMS, % | 42 (67.6) | 23 (69.7) | 19 (65.5) | |
| DES and BMS, % | 2 (3.2) | 1 (3.0) | 1 (3.5) | |
| Staged procedureb | 10 (14.5) | 5 (14.7) | 5 (14.3) | .61 |
| Periprocedural “complications” | 10 (14.3) | 9 (25.7) | 1 (2.9) | .01 |
| Cardiogenic shock | 3 | 3 | 0 | |
| Apical thrombus | 1 | 1 | 0 | |
| Total AV block | 2 | 2 | 0 | |
| Periprocedural MI | 1 | 1 | 0 | |
| Hematoma | 3 | 2 | 1 | |
| Initial TIMI flow grade | .22 | |||
| 0-1 | 37 (52.9) | 22 (62.9) | 15 (42.9) | |
| 2 | 16 (22.9) | 7 (20.0) | 9 (25.7) | |
| 3 | 17 (24.3) | 6 (17.1) | 11 (31.4) | |
| Final TIMI flow grade post PPCIb | ||||
| 0-1 | 1 (1.4) | 0 (0.0) | 1 (2.9) | .37 |
| 2 | 1 (1.4) | 0 (0.0) | 1 (2.9) | |
| 3 | 67 (97.1) | 34 (100.0) | 33 (94.3) |
AV, atrioventricular; BMS, bare metal stent; DES, drug eluting stent; GP, glycoprotein; MI, myocardial infarction; PPCI, primary percutaneous coronary intervention; TIMI, Trombolysis In Myocardial Infarction.
Data are expressed as No. (%) or mean ± standard deviation.
Percentages are calculated based on known values.
Four weeks to 6 weeks after PPCI, endothelial dysfunction (RH-PAT index<1.67) was found in 35 patients (50%); the other 35 patients (50%) showed normal endothelial function (RH-PAT index ≥ 1.67). In the group with endothelial dysfunction, the mean RH-PAT index measured 1.48±0.12 (range 1.10-1.65). In patients with normal endothelial function (RH-PAT ≥ 1.67), the mean RH-PAT was 2.31±0.56 (range 1.67-3.63).
Between these 2 patient groups, there was no difference in: demographics and patient characteristics, type of stents (eg, drug-eluting stent use), frequency of staged procedures and many other procedure-related parameters, and medication at discharge (Table 2). Significant between-group differences were a shorter total ischemia time, more periprocedural complications such as cardiogenic shock, and a greater use of glycoprotein IIb/IIIa receptor antagonists in patients with endothelial dysfunction (Table 2).
Long-term Follow-up and Major Adverse Cardiovascular EventsThe mean duration of follow-up for MACE was 4.0±1.7 years until the (first) MACE or event-free survival. Of the 70 patients, 50 remained event-free, while 20 (28.6%) experienced MACE. Four patients died, of whom 2 had endothelial dysfunction and 2 had normal endothelial function (P=.69). Of 13 patients who underwent coronary revascularization procedures, 7 had endothelial dysfunction and 6 had normal endothelial function (P=.50). Two patients had a stroke and both had a normal endothelial function (P=.25). Of the 13 patients who underwent coronary revascularization procedures, acute myocardial infarction was the reason in 2 patients with endothelial dysfunction and in none of the patients with normal endothelial function (P=.25). Treatment for newly developed peripheral arterial disease was required in 3 patients with normal endothelial function and in none of the patients with endothelial dysfunction (P=.12).
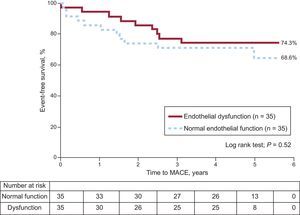
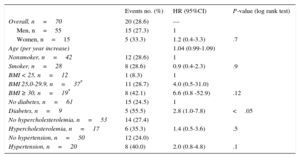
Endothelial Dysfunction, Traditional Cardiovascular Risk Factors, and Clinical OutcomeDuring follow-up, MACE occurred in 9 of 35 (25.7%) patients with endothelial dysfunction and in 11 of 35 (31.4%) patients with normal endothelial function (P=.52) (Figure). There was no significant difference in MACE risk between patients with endothelial dysfunction and patients with normal endothelial function (hazard ratio=0.8; 95% confidence interval, 0.3-1.8). Ten out of 51 (19.6%) patients with single vessel disease developed MACE compared with 9 of 18 (50%) patients with multivessel disease (P<.05), but the presence of multivessel disease did not show a significant relationship with endothelial dysfunction (P=.17). Of the traditional cardiovascular risk factors, diabetes mellitus (hazard ratio=2.8; 95% confidence interval, 1.0-7.8) showed an association with MACE on univeriate analysis (Table 3); even in multivariate analyses the risk appeared to be increased although not significantly (hazard ratio=2.5; 95% confidence interval, 0.8-7.5) (Table 4).
Major adverse cardiovascular event-free survival of patients with endothelial dysfunction vs normal endothelial function. During a follow-up of 4.0±1.7 years, 50 patients (of a total of 70 patients) experienced a major adverse cardiovascular event. There was no significant difference in the risk of a major adverse cardiovascular event between patients with normal endothelial function vs dysfunction (hazard ratio=0.8; 95% confidence interval, 0.3-1.8). MACE, major adverse cardiac events.
Association Between Cardiovascular Risk Factors and Major Adverse Cardiovascular Events
| Events no. (%) | HR (95%CI) | P-value (log rank test) | |
|---|---|---|---|
| Overall, n=70 | 20 (28.6) | — | |
| Men, n=55 | 15 (27.3) | 1 | |
| Women, n=15 | 5 (33.3) | 1.2 (0.4-3.3) | .7 |
| Age (per year increase) | 1.04 (0.99-1.09) | ||
| Nonsmoker, n=42 | 12 (28.6) | 1 | |
| Smoker, n=28 | 8 (28.6) | 0.9 (0.4-2.3) | .9 |
| BMI < 25, n=12 | 1 (8.3) | 1 | |
| BMI 25,0-29,9, n=37* | 11 (28.7) | 4.0 (0.5-31.0) | |
| BMI ≥ 30, n=19* | 8 (42.1) | 6.6 (0.8 -52.9) | .12 |
| No diabetes, n=61 | 15 (24.5) | 1 | |
| Diabetes, n=9 | 5 (55.5) | 2.8 (1.0-7.8) | <.05 |
| No hypercholesterolemia, n=53 | 14 (27.4) | ||
| Hypercholesterolemia, n=17 | 6 (35.3) | 1.4 (0.5-3.6) | .5 |
| No hypertension, n=50 | 12 (24.0) | ||
| Hypertension, n=20 | 8 (40.0) | 2.0 (0.8-4.8) | .1 |
95%CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
Multivariate Analysis: the Risk of Endothelial Function on Major Adverse Cardiovascular Events, Adjusted for Cardiovascular Risk Factors
| Endothelial function (dysfunction vs normal) | HR (95%CI) |
|---|---|
| Crude HR | 0.8 (0.3-1.8) |
| Adjusted for age (continuous) and sex | 0.8 (0.3-2.0) |
| Adjusted for diabetes at baseline | 0.6 (0.3-1.6) |
| Adjusted for BMI (categorical) at baseline | 0.9 (0.4-2.3) |
| Adjusted for diabetes and BMI (categorical) | 0.8 (0.3-2.0) |
| Adjusted for age and sex, diabetes and BMI (categorical) | 0.8 (0.3-2.0) |
95%CI, 95% confidence interval; BMI, body mass index; HR, hazard ratio.
While endothelial dysfunction is a key component of atherogenesis, contributes to the development of cardiovascular disease, and predicts outcome in patients with or without overt coronary artery disease,1–7 its significance for the highest risk category—patients with STEMI—was less certain. In the present prospective cohort study in STEMI patients of the RESPONSE trial that involved medical treatment according to evidence-based pharmacological concepts,24 endothelial dysfunction was associated with more acute periprocedural “complications” (eg, cardiogenic shock, total atrioventricular block, left ventricular thrombus) but showed no relationship to long-term clinical outcome after PPCI. The MACE rate was similar in patients with endothelial dysfunction vs patients with normal endothelial function. The findings disprove our initial hypothesis that endothelial dysfunction may predict long-term clinical outcome following PPCI for STEMI.
Patients with diabetes mellitus at baseline had a significantly higher MACE rate during follow-up. Even on multivariate analyses the risk appeared to be increased, although not significantly, which could be related to the limited number of patients. In addition, we cannot exclude the possibility that some initially nondiabetic patients may have developed (yet undetected) diabetes during the 4 years of follow-up. Nevertheless, our data suggest that the traditional cardiovascular risk factor, diabetes, together with other risk factors, may be prognostically important even in a population known to have increased cardiovascular risk.32 The findings of the present study may imply that in STEMI patients, representing the highest disease stage of coronary atherosclerosis,8,33 the total extent of vessel wall changes (ie, plaque volume) and the number and vulnerability of atherosclerotic lesions (ie, plaques prone to rupture), which are both known to be increased in patients with diabetes,33 might be more important for overall cardiovascular risk than endothelial dysfunction.
Previous Studies on Endothelial Dysfunction and OutcomeSuwaidi et al10 were among the first to demonstrate that patients with nonobstructive coronary artery disease and severe endothelial dysfunction (invasively assessed with acetylcholine) had an increased cardiovascular event risk during a mean follow-up of 28 months. The presence of coronary endothelial dysfunction in the absence of obstructive coronary disease was also shown to be independently associated with an increased cerebrovascular risk.13 Schächinger et al11 demonstrated through invasively assessed endothelial function that significantly impaired endothelium-dependent epicardial coronary vasoreactivity is an independent predictor of future cardiovascular events.
Other research groups assessed peripheral endothelial function noninvasively by measuring the flow-mediated dilatation of the brachial artery and found that endothelial dysfunction independently predicted long-term cardiovascular outcome in patients with peripheral arterial disease.12 In 2 studies, endothelial dysfunction, as assessed with flow-mediated dilatation, showed a significant relationship with in-stent restenosis following PCI14,15; patients with endothelial dysfunction also showed more cardiovascular events during 12 months follow-up.15 Guazzi et al17 found in patients with uncomplicated myocardial infarction—predominantly non—ST-segment elevation myocardial infarction (80%)—an increased cardiovascular event risk during an average follow-up of 14 months, if patients had a combination of both endothelial dysfunction and diabetes mellitus.
Only a single study by Wang et al18 also examined the potential impact of endothelial dysfunction on clinical outcome following STEMI. In that study, which had a 1-year follow-up, endothelial dysfunction independently predicted cardiovascular events in addition to diabetes mellitus and left ventricular ejection fraction.18 In our present study, diabetes was also identified as a significant predictor of adverse events, but endothelial function did not predict clinical outcome. Our study differed in many ways from the aforementioned Chinese study. Several of these dissimilarities may have contributed to the difference in findings. The main differences between the 2 studies are the predominantly Caucasian vs Chinese patient populations, a mean follow-up of 4 years vs 1 year, the use of the RH-PAT vs the flow-mediated dilatation method, the use of nitrates (87% of the Chinese patients were on nitrates), and the timing of endothelial function measurement on 28 to 42 days vs 5 days after STEMI. That early timing of endothelial function measurement in the study by Wang et al implies a risk of a significant disturbance from the inflammation that is associated with “myocardial repair” following STEMI, a process that is largely finished after 3 weeks, which comes close to the time that endothelial function measurements were performed in the present study. An improvement of endothelial function following PCI in patients with non—ST-segment elevation myocardial infarction has previously been demonstrated.34
LimitationsBecause of the limited size of the study population and the number of events, our findings should be interpreted with caution; nevertheless, many other studies of endothelial function also evaluated relatively small study populations.10,17 The present pilot study does not allow conclusions to be drawn on patients who did not survive the first 4 weeks to 6 weeks after STEMI or whose status prevented their enrollment. The system used for endothelial function measurements might not be ideal to identify endothelial dysfunction in patients with a recent STEMI. In addition, the presence of untreated lesions with diameter stenoses ≥ 50% at the index procedure could have had an impact on the occurrence of MACE, and MACE may not be an optimal clinical endpoint in this context. Reproducibility studies with RH-PAT have previously been reported, but were not performed by the local study laboratory. Future large-scale studies with other techniques to evaluate the potential impact of endothelial dysfunction after PPCI on clinical outcome may still be warranted.
In addition, the RESPONSE study assessed the impact of nurse-led secondary prevention clinics vs standard care on cardiovascular risk.24 We cannot exclude the possibility that healthcare providers may have paid more attention to an optimal lifestyle modification and the prescription of drug therapy according to current guidelines. Moreover, the compliance of the trial participants may have been higher than average. As a result, medical treatment and endothelial function might have been somewhat better than in everyday patients.
Endothelial function measurements were performed after 4 weeks to 6 weeks, when all patients were treated with a similar secondary preventive medication that included statins and frequently ACE inhibitors, which may have had an overall favorable effect on endothelial function in this study population.35–38 Nevertheless, there was no significant difference in medical treatment between patients with vs without endothelial dysfunction. However, medication was recorded at discharge and not at endothelial function measurement.
In fact, coronary endothelial function following STEMI has a dynamic nature and may be impaired during the first few weeks after a STEMI. It was previously shown to improve between day 9 and 1-year follow-up in patients who underwent thrombolytic therapy,39 but endothelial dysfunction may be smaller in patients who were treated by PPCI.40 Inflammation and repair processes of the infarcted myocardium, which may disturb endothelial function during the first few weeks, are completed 4 weeks to 6 weeks after PPCI.25–27 Consequently, most changes in endothelial function can be assumed to be finished after 4 weeks to 6 weeks. As the vast majority of MACE occurred after that point in time, our present endothelial function measurements and the assessment of a potential relationship with future MACE should be clinically relevant. Nevertheless, we cannot exclude the possibility that the assessment of endothelial function at a single point in time may not reflect endothelial function over the entire period of follow-up. To account for the dynamic nature of endothelial function, further serial data on endothelial function after STEMI would be of great interest.
Implications of the StudyThe present study, with an average follow-up of 4 years, presents unique data on endothelial function and the long-term clinical outcome of STEMI patients treated with PPCI. Our findings suggest that RH-PAT-based assessment of endothelial function (as a potential surrogate marker of cardiovascular risk) may be of limited value in patients with recent STEMI. In these patients, traditional risk factors (such as diabetes) might be more relevant for clinical outcome than endothelial dysfunction.
CONCLUSIONSIn this series of patients who survived a STEMI, endothelial dysfunction, as assessed by RH-PAT 4 weeks weeks to 6 weeks from PPCI, did not predict future MACE during an average follow-up of 4 years. Future large-scale studies with other techniques to assess endothelial dysfunction after PPCI may still be warranted.
CONFLICTS OF INTERESTThe research department of Thoraxcentrum Twente has received research grants funded by AstraZeneca, Biotronik, Boston Scientific, and Medtronic. C. von Birgelen has been a consultant for Boston Scientific and Medtronic, and has received lecture fees from AstraZeneca and MSD. R. J. G. Peters has been a consultant for Amgen, AstraZeneca, and Sanofi, and has received lecture fees from AstraZeneca, Boehringer Ingelheim, and Sanofi.