More than 140 million people dwell at altitudes of over 2500 m. The Andes region is home to the greatest population density at an altitude of more than 3500 m. Equally, the number of persons living at sea level (SL) is growing, and they travel to medium, high, or extreme altitudes for occupational or scientific reasons, or to enjoy tourism or recreational activities, as is the case of hikers, mountaineers, and skiers. For this reason, it is interesting to determine the effects of altitude on physiological and structural aspects of the human body.
The effects of altitude are due to the low barometric pressure and, thus, to a reduction in the partial pressure of inspired oxygen. This condition of hypobaric hypoxia is what causes alveolar hypoxia and hypoxemia in humans who live at or ascend to high altitudes. There are multiple responses to the hypoxic stimulus, as well as numerous mechanisms of acclimatization. On occasions, an individual who ascends to high altitudes adapts poorly, whereas in other cases, a high-altitude native loses acclimatization. This editorial provides a brief review of the effects of altitude on the pulmonary circulation in three situations: chronic hypoxia, acute hypoxia, and subacute hypoxia.
Chronic hypoxia High-Altitude Natives in Normal HealthPioneering studies, carried out by Peruvian researchers using cardiac catheterization, demonstrated the presence of asymptomatic pulmonary hypertension (PH) in healthy native residents of an Andean community located at an altitude of 4540 m.1 Subsequent investigations performed at different altitudes in the Andes and in Asia found that the relationship between altitude and mean pulmonary artery pressure (mPAP) is represented by a parabolic curve that shows that, above 3500 m, mild to moderate PH develops in association with adaptive levels of hypoxemia and polycythemia. In our study, at 4540 m (barometric pressure, 445mmHg; partial pressure of inspired oxygen, 80mmHg), the mPAP was 28mmHg, with an arterial oxygen saturation (SaO2) of 80% and hemoglobin at 20g/dL.1 In natives living at this altitude who died in accidents, quantitative histological study of the pulmonary arteries demonstrated thickening of the medial smooth muscle cell (SMC) layer in the small distal arteries, with extension of the muscle layer to the arterioles.2 These findings explain the increases in pulmonary vascular resistance, PH, and right ventricular hypertrophy.
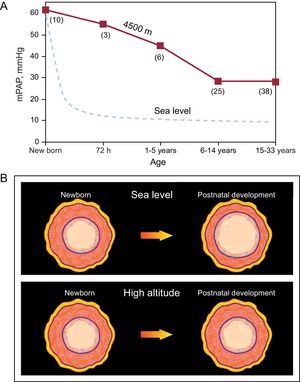
In a historical review, Reeves and Grover3 noted that Peruvian scientists, in their description of postnatal changes in pulmonary circulation, were the first to demonstrate the pathogenesis of chronic hypoxic PH in humans.3 The Peruvian researchers showed that newborns at high altitudes (HA) have PH and a thick SMC layer in the small arteries and arterioles of the lungs, findings similar to those described at SL. These findings constitute, both at SL and at HA, a residual expression of the fetal pattern. However, newborns at SL undergo rapid vascular remodeling—thinning of the muscle layer with enlargement of the lumen—a process that leads to a rapid decrease in pulmonary vascular resistance and PAP. In contrast, in newborns at HA, the vascular remodeling occurs slowly over the course of his or her lifetime; thus, PH and right ventricular hypertrophy persist into adulthood (Figure 1).1, 2, 4
Figure 1. A: relationship between mean pulmonary arterial pressure and age in natives with normal health who live at a high altitude, at 4540 m (solid line), compared to the data reported for sea level residents (dashed line) (the numbers in parenthesis indicate the number of cases); the mean pulmonary arterial pressure decreases rapidly at sea level; in contrast, in high-altitude natives, the grade of pulmonary hypertension decreases slowly and can persist into adulthood. B: illustration of postnatal remodeling of distal pulmonary arteries; this maturation process occurs rapidly at sea level, in contrast with the slow process that takes place at high altitudes. mPAP, mean pulmonary arterial pressure. Reproduced from Penaloza and Arias-Stella 4 with the permission of Wolters Kluwer-LWW.
During exercise, the response of the PAP in HA natives is greater than in residents at SL, even though cardiac workload and output are similar or even lower. This is because of the lesser distensibility of the pulmonary arteries as a consequence of the thick SMC layer.5 However, HA natives are capable of performing physical activity similar to or even greater than that of residents at SL acclimated to HA. The PH of HA natives is reversible after the person has lived for a long period at SL.6 Interestingly, the behavior of Tibetan natives, who have the longest lines of highland ancestors, is similar to that of SL residents both at rest and during physical activity because they are genetically adapted.7
Chronic Mountain SicknessSome residents of HA lose their capacity for adaptation and develop chronic mountain sickness (CMS).8 These patients undergo a reduction in alveolar ventilation, which is more pronounced when they are sleeping. Alveolar hypoventilation provokes a higher degree of hypoxemia in natives with normal health. Consequently, there is an increased response to erythropoietin, exaggerated polycythemia, marked PH, an increase in right ventricular hypertrophy and, in some cases, congestive heart failure. In a hemodynamic study carried out by our group in patients with CMS, the following data were obtained: mPAP, 47mmHg; SaO2, 70%; and hemoglobin, 25g/dL.4, 9
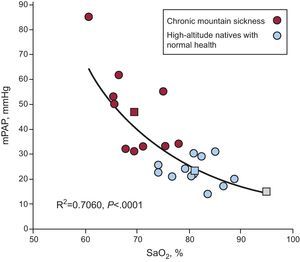
The clinical picture in these patients is similar to that observed at SL in cases of advanced chronic obstructive pulmonary disease, superobesity, and severe sleep apnea. An important observation in CMS is the inverse relationship between mPAP and SaO2. The lower the SaO2, the higher the PH. This is shown in Figure 2, which compares patients with MMC, healthy HA natives and SL residents.4 During exercise, even light exercise, a decrease in SaO2 and severe PH are observed.4, 9, 10 During sleep, there is marked hypoventilation and hypoxemia and, consequently, exaggerated PH develops.11
Figure 2. Inverse relationship between mean pulmonary arterial pressure (mPAP) and arterial oxygen saturation in patients with chronic mountain sickness. As the arterial oxygen saturation decreases, the mean pulmonary arterial pressure increases; thus, the patient with the lowest arterial oxygen saturation has the highest grade of hypertension (red). For the purpose of comparison, the values obtained in natives with normal health living at high altitudes are shown (blue). The mPAP levels corresponding to these 2 groups and to sea level residents are also shown (squares). mPAP, mean pulmonary arterial pressure; SaO2, arterial oxygen saturation. Reproduced from Penaloza and Arias-Stella 4 with the permission of Wolters Kluwer-LWW.
CMS can be primary, due to alveolar hypoventilation related mainly to aging, and is frequently secondary to lung disease, obesity, and sleep apnea. In other cases, CMS is related to smoking and contamination, both environmental (mining towns) and domestic (chronic exposure to smoke from stoves that burn wood or other biomass fuels). The few pathology reports in patients who died with a diagnosis of CMS and heart failure correspond to secondary CMS. Among the findings in these cases are marked right ventricular hypertrophy, excessive muscularization of the distal pulmonary arteries, and adventitial thickening.4
The ideal treatment of CMS is descent to SL. A palliative alternative is isovolemic phlebotomy. Drug trials have been performed with acetazolamide, which increases alveolar ventilation and improves oxygenation of the blood; nifedipine, which reduces PH but has a systemic effect; and sildenafil, which selectively reduces PH and improves exercise tolerance.4
Acute hypoxia Acute Mountain SicknessSL residents who ascend to medium or high altitudes develop some degree of acute mountain sickness or soroche. The degree of susceptibility to acute mountain sickness varies. The most common symptoms are headache, sleep disorders, gastrointestinal disorders, and dizziness. The major cause is hypoxemia and, thus, the treatment is oxygen administration and, in severe cases, a return to lower altitudes. Interestingly, ascent to higher altitudes increases PAP, both in individuals with acute mountain sickness and in those who remain asymptomatic after the climb. This is an intrinsic response of SMC to alveolar hypoxia; the degree of PH is generally mild and does not contribute to the symptoms of acute mountain sickness. It can occur in tourists, as well as in hikers, skiers, and mountaineers. Acetazolamide administration is the most widely accepted prophylaxis. Staging the ascent attenuates the degree of PH12 and, thus, is recommended as a way to prevent a serious clinical condition known as high-altitude pulmonary edema (HAPE) associated with severe PH.
High-Altitude Pulmonary EdemaSome SL residents who ascend to HA develop HAPE, a serious and potentially fatal clinical entity. This condition occurs a few days after arriving at the HA. The first cases were reported in Peru, in a number of young HA natives who were returning to the elevated region after spending a few weeks at SL.13 This clinical form is referred to as reentry HAPE. Since then, numerous studies have been performed in the Andes, in the Rocky Mountains in the Unites States, in Asia, and in the Alps. In Monte Rosa, located in the Italian Alps at an altitude of 4559 m, important experimental studies have been carried out on susceptibility to HAPE and its mechanism, prevention, and treatment.
HAPE is a noncardiogenic form of pulmonary edema due to severe hypoxic PH and an increase in alveolar capillary membrane permeability. Factors that determine the development of HAPE are the altitude, the rate of ascent, the degree of physical activity, and individual susceptibility. The clinical picture is characterized by fatigue, dyspnea, cough, pink frothy sputum, tachycardia, cyanosis, and pulmonary rales. Chest radiography shows infiltrates in the form of dense, confluent opacities with variable distribution throughout the lung fields.14 HAPE develops in susceptible individuals with exaggerated pulmonary arterial reactivity to hypoxia. Susceptibility has been attributed to a defect in pulmonary nitric oxide synthesis.15
There are few hemodynamic studies involving cardiac catheterization during an episode of HAPE at the same altitude at which the event occurred.16 Peruvian researchers performed studies of this type in a laboratory located in the Andean community of Cerro de Pasco, situated at an altitude of 4340 m, and detected the lowest SaO2 levels (mean, 57%) and the most severe PH (mean mPAP, 63mmHg) ever reported in HAPE.17 Maggiorini et al.18 carried out similar studies in susceptible mountaineers in whom they induced HAPE experimentally by means of a rapid ascent to a laboratory built in the “Regina Margherita” hut, at the top of Monte Rosa, at 4559 m. In this study, the authors demonstrated the increase in pulmonary capillary pressure (mean, 22mmHg) and normal pulmonary artery occlusion pressure (or wedge pressure).18 The current concept of the pathogenesis of HAPE implies the combination of 2 concurrent processes: a) exaggerated and uneven hypoxic pulmonary vasoconstriction, which leads to an excessive elevation in blood pressure and an increase in pulmonary capillary pressure and, consequently, alveolar capillary membrane damage (capillary stress failure) and alveolar flooding18, 19, 20, and b) reduced alveolar fluid clearance due to impaired transepithelial sodium transport.21
The mechanism of PH in HAPE differs from that described in HA natives, in whom the major factor in PH is the thick SMC layer in the distal pulmonary arteries. In most cases of HAPE, the major factor of severe PH is not structural, as it occurs in SL residents with normal pulmonary arteries (tourists, hikers, mountaineers). The major factor is hypoxic vasoconstriction, a property intrinsic to SMC. The hypoxic stimulus acts in the SMC sarcolemma, inhibiting the outward current in the voltage-gated K+ (Kv) channels, a circumstance that induces membrane depolarization and opening of the voltage-gated Ca2+ channels. The influx of Ca2+ stimulates the release of Ca2+ deposited in the sarcoplasmic reticulum, resulting in an increase in cytosolic Ca2+ which, through a series of enzymatic events, acts on the actomyosin contractile apparatus and induces SMC contraction.22
However, acute hypoxia also induces endothelial dysfunction, which activates the endothelin pathway and its vasoconstrictor effect,23 as it depresses the nitric oxide and prostacyclin pathways and their vasodilator effect. In addition, acute hypoxia activates the sympathetic nervous system and its vasoconstrictor effect.24 These mechanisms explain the beneficial effect of oxygen, nitric oxide, and vasodilators such as Ca2+ channel blockers (nifedipine), prostacyclin analogues (iloprost), and phosphodiesterase-5 inhibitors (sildenafil, tadalafil). Depending on the agent employed, the Ca2+ channels are blocked, the K+ channels are opened, or the nitric oxide and prostacyclin pathways are activated, an action that increases the generation of cyclic guanosine monophosphate and cyclic adenosine monophosphate, mediators of SMC vasorelaxation.
Subacute hypoxiaThe effects of subacute hypoxia on the heart and pulmonary circulation were described 5 decades ago in calves taken to graze at altitudes of between 2500 and 3770 m in the Rocky Mountains in Utah (United States). After a few weeks, the animals experienced an excessive elevation in blood pressure in association with a thick SMC layer in the distal pulmonary arteries and, consequently, exaggerated right ventricular hypertrophy and heart failure. These animals died if they remained at high altitude, but recovered rapidly once taken to lowlands. This disease produced in cattle by subacute hypoxia is referred to as brisket disease.25
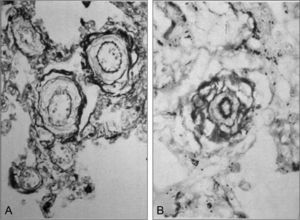
Five decades ago, Chinese researchers discovered a similar clinical picture in infants of the Han ethnic group who, after being born at low altitudes, were taken to HA where, in a matter of weeks or months, they developed severe PH and heart failure, which proved fatal if the infants were not taken to lowlands. Years later, Chinese and British researchers performed a clinical and pathological study and referred to this entity as subacute infantile mountain sickness. 26, 27 A notable finding of this study is the massive right ventricular hypertrophy and exaggerated hypertrophy of the medial SMC layer in the small arteries and arterioles of the lung (Figure 3), which implies excessive vasoreactivity in response to hypoxia, as well as severe PH. The vascular origin of subacute infantile mountain sickness contrasts with the initial respiratory mechanism observed in CMS in the adult. Hemodynamic studies of subacute infantile mountain sickness performed with cardiac catheterization and, more recently, with Doppler ultrasound, have confirmed the presence of severe PH. In a study carried out in 55 children in China, the mPAP reached 72mmHg.28 Subacute infantile mountain sickness is observed less frequently in the Andes, but a few cases have been reported.2
Figure 3. A: cross-sections of small pulmonary arteries of an 11-month-old infant of the Han ethnic group whose family moved to Llasa, Tibet, at an altitude of 3600 m; after a few weeks, he developed subacute infantile mountain sickness; marked hypertrophy of the medial muscle layer can be observed (Elastica van Gieson×600). B: cross-section of an arteriole of the same infant; a very thick medial muscle layer is observed between the elastic lamellae, with a particularly thick internal layer (Elastica van Gieson×1000); the fact that, at sea level, the arterioles have no muscle layer and their 2 elastic lamellae are fused should be taken into account. Reproduced from Heath, 27 with the permission of the Universidad Peruana Cayetano Heredia.
Conflicts of interestNone declared.
Corresponding author: Instituto de Investigaciones de la Altura, Universidad Peruana Cayetano Heredia, Avda. Honorio Delgado 430, Urb. Ingeniería, S.M.P., Lima 31, Peru. penaloza.dante@gmail.com