Chagas is an endemic disease in Latin America, caused by the parasite Trypanosoma cruzi, which usually affects the functioning of the heart. We have studied the regulation of intracellular calcium in cardiomyocytes isolated from chagasic patients with different degrees of heart dysfunction.
MethodsCalcium selective microelectrodes were used to simultaneously measure diastolic calcium concentration ([Ca2+]d) and resting membrane potential in endomyocardial biopsies obtained from chagasic patients and controls.
ResultsThe [Ca2+]d increased by 123%, 295%, and 738% in chagasic patients in functional class I, II, and III, respectively, in relation to controls. Membrane potential showed a partial depolarization of 6% in functional class I, 10% in functional class II, and 22% in functional class III, compared to control values. Alteration in the [Ca2+]d was partially reverted by 1-[6-[[(17ß)-3-metoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U-73122), a β-phospholipase C antagonist, and by 2-aminoethoxydiphenyl-borate (2-APB), an inositol 1,4,5-trisphosphate receptor blocker. Phenylephrine, an agent that induces a rapid transient increase in 1,4,5-trisphosphate intracellular content, produced a rise in [Ca2+]d, higher in chagasic cardiomyocytes than in controls, and its effect was fully inhibited by 2-APB.
ConclusionsIn cardiomyocytes from chagasic patients there is a dysfunction of the regulation of the [Ca2+]d, which correlates with the cardiac abnormalities observed in the different stages of the disease. This disturbance in the regulation of intracellular calcium appears to be associated with alterations in the regulation of intracellular messenger inositol 1,4,5-trisphosphate.
Keywords
Of the 80 different pathologies that are referred to as “tropical,” Chagas disease represents the most important health issue on the American continent.1 This parasitemia is produced by a hemoflagellate, Trypanosoma cruzi, which primarily, although not exclusively, affects the heart, producing lesions that are clinically referred to as chagasic cardiomyopathy. This disease tends to progress in time through 3 phases: acute, indeterminate, and chronic, with the last phase being the most common clinical and pathological type.2 The chronic phase is initially expressed as a nondilated cardiomyopathy, which slowly progresses into dilated cardiomyopathy, along with arrhythmia, left ventricular systolic and diastolic dysfunction, thromboembolic episodes, and sudden death.3
Previous studies performed with noncardiac tissues demonstrated a possible link between chagasic infections and altered regulation of intracellular calcium levels and the intracellular messenger inositol 1,4,5-trisphosphate.4, 5 However, these studies were carried out with animal models, and the information obtained from them cannot necessarily be extrapolated to explain what occurs in human cardiomyocytes of patients with Chagas disease. This study investigated whether or not chagasic cardiomyopathy is associated with changes in diastolic calcium concentration ([Ca2+]d) in endomyocardial biopsies of chagasic patients. Additionally, we studied the role of the intracellular messenger inositol trisphosphate.
Methods Population StudiedThis study was carried out with 15 chagasic patients (4 women and 11 men), with an average age of 46±1.6 years. Patients were grouped according to the New York Heart Association (NYHA) classification system, which takes into account the patient's clinical manifestations and risk factors that affect mortality: early (functional class [FC] I), intermediate (FCII), and late (FCIII). Based on this classification system, 4 patients fell within FCI, 6 in FCII, and 5 in FCIII. All chagasic patients who participated in this study had a positive epidemiological history, an abnormal electrocardiogram at rest (ventricular arrhythmia, right branch block), and positive blood culture and enzyme-linked immunosorbent assay (ELISA) results for Chagas disease. Of the 15 patients studied, 11 had received conventional treatment (amiodarone, digitalis, and diuretics) (Table 1).
Table 1. Number of Cardiomyocytes Obtained per Patient for the Measurements of Membrane Potential and [Ca2+]d.
| Patients | Control | FCI | FCII | FCIII |
| 1 | 10 | 10 | 5 | 3 |
| 2 | 6 | 6 | 5 | 2 |
| 3 | 10 | 10 | 6 | 4 |
| 4 | 4 | 3 | 4 | 0 |
| 5 | 0 | 0 | 5 | 0 |
| 6 | 0 | 0 | 3 | 0 |
[Ca2+]d, diastolic calcium concentration; FC, functional class.
Ten patients (4 women and 6 men, with a mean age of 32±1.5 years) who suffered from mitral regurgitation or failure were included as the control group. None of these patients showed any symptoms of Chagas disease.
Endomyocardial Biopsy of the Left VentricleEndomyocardial biopsy samples were obtained from the chagasic patients using fluoroscopic control and from control patients during mitral valve replacement surgery, attempting to obtain the tissue from the same area in both patient groups. Although not all patients included in this study were taking medications at that time, those who were suspended medication 48h before the endocardial biopsies. The biopsied tissues were immediately placed in Tyrode's solution with a low Ca2+ concentration, which was supplemented with 20mM of 2,3-butanedione monoxime, a potent inhibitor of actin-myosin interaction, to prevent mechanical activation. A mix of O2 (95%) and CO2 (5%) was also bubbled through the solution at 37 oC. The connective tissue was dissected, allowing for small segments of the left ventricle to be obtained, each with 10 to 15 cardiomyocytes. The cardiomyocytes were placed in an experimental chamber, which was perfused with Tyrode's solution (37oC), and the same mix of O2 (95%) and CO2 (5%) was passed through it. The muscle tissue sample was then pinned to the floor of the experimental chamber in order to measure [Ca2+]d. All biopsies were obtained with patient consent and approval from the bioethics committee of the respective hospitals.
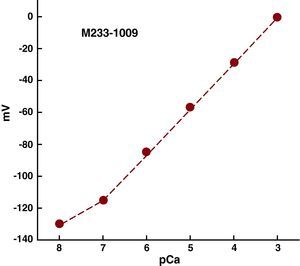
Double-Barrelled Calcium Selective MicroelectrodesDouble-barrelled glass capillaries (WPI-PB150F-4, Sarasota, Florida) were washed with hydrochloric acid and then with distilled water. They were dried in an oven (200°C) and stretched in a pipette puller (P-97 Flaming/Brown, Automate Scientific, Berkeley, California) to obtain small-tipped microelectrodes (<1μM). The microelectrodes were then prepared and calibrated as previously described.6 Only those microelectrodes with a Nernst type response (30.5mV per unit pCa at 37°C) were used in the experiment (Figure 1).
Figure 1. Calibration curve of a calcium selective microelectrode. The y-axis displays electric potential as measured by the calcium microelectrode, and the x-axis displays the various calcium concentrations (pCa3-pCa8), in which pCa=-log[Ca2+]. A Nernst-type response was observed (30.5mV per unit pCa at 27°C) within the range of pCa3 to pCa7. The resistance of the microelectrodes was 5.2 × 1011Ω.
Quantification of Intracellular Calcium ConcentrationsThese experiments were performed within 3h after the biopsy. The cardiomyocytes were impaled individually by the double-barrelled Ca2+ sensitive microelectrode. We only considered those measurements registered from control and chagasic cardiomyocytes with stable potentials (membrane potential [Vm] and specific calcium potential [VCae]) for no less than 40seconds. All microelectrodes were recalibrated after each [Ca2+]d measurement, and if the two calibration curves (pre- and post-measurement) were more than 3mV apart within the pCa6 - pCa7 range, the measurement was rejected.6
The electric potential of Vm and calcium potential (VCa) was measured using a high-impedance amplifier (WPI FD-223, Sarasota, Florida). VCae was obtained by subtracting the initial VCa value from the Vm. All measurements of electric potential were stored in a computer (Macintosh MA878LL/ACupertino, California) for later analysis.
SolutionsThe Tyrode's solution was made up of the following components (mM): 130 NaCl, 3 KCI, 1.8 CaCI2, 1 MgCI2, 12 NaHCO3, 5 glucose, 4 (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and the pH was adjusted to 7.4 using NaOH. The low-calcium solution had the following composition (mM): 130 NaCl, 3 KCI, 1 MgCI2, 5 sodium pyruvate, 20 glucose, 20 2,3-butanedione monoxime, 10 HEPES, 6 nitrilotriacetic acid, and the pH was adjusted to 7.4.
StatisticsThe data were expressed as mean±standard deviation, and n was the number of ventricular cardiomyocytes in which the [Ca2+]d was determined. The difference was assessed for statistical significance using a one-way analysis of variance. Tukey test was used for multiple comparisons, and t-test for paired data. P was considered significant when less than .05.
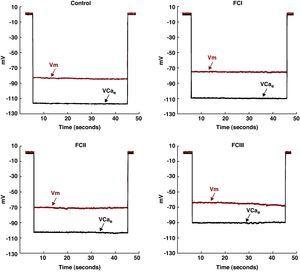
Results Diastolic Intracellular CalciumThe values of Vm and [Ca2+]d obtained from the chagasic patients’ ventricular cardiomyocytes were grouped according to NYHA functional class. In the cardiomyocytes isolated from chagasic patients, we observed a partial depolarization of the cell membrane associated with an increase in [Ca2+]d in comparison with the controls (Figure 2). These changes appeared to be associated with the severity of the disease.
Figure 2. Simultaneous measurements of Vm and [Ca2+]d in cardiomyocytes from control patients and chagasic patients in functional classes I, II, and III. The control values were Vm: -84mV and [Ca2+]d: 118nM. The values from functional class I cells were Vm: -75mV and [Ca2+]d: 233nM. The values from functional class II cells were Vm: -70mV and [Ca2+]d: 387nM. The values from functional class III cells were Vm: -65mV and [Ca2+]d: 950nM. [Ca2+]d, diastolic calcium concentration; FC, functional class; Vcae,: specific calcium potential; Vm, membrane potential.
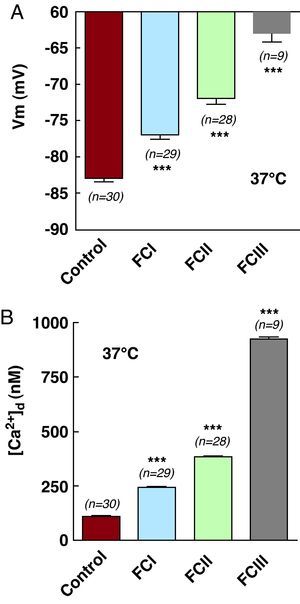
In cardiomyocytes obtained from healthy patients at-rest, the mean Vm was -83±2.3mV (n=30), and mean [Ca2+]d was 111±4.4nM (n=30) (Table 1 and Figure 3A and 3B), whereas those obtained from FCI chagasic patients had a mean Vm of -77±3mV (n=29), P<.001, compared to control values (Figure 3A), and mean [Ca2+]d was 245±12nM (n=29), P<.001 (Figure 3B). The mean Vm of FCII patients was -72±4mV (n=28), P<.001 (Figure 3A), and mean [Ca2+]d was 385±12nM (n=28), P<.001 (Figure 3B). In ventricular cells obtained from FCIII patients, mean Vm was 63±3.5mV (n=9), P<.001 (Table 1 and Figure 3A), and [Ca2+]d was 922±33nM (n=9), P<.001 (Table 1 and Figure 3B).
Figure 3. Simultaneous measurements of (A) membrane potential and (B) [Ca2+]d for control, functional class I, functional class II, and functional class III cardiomyocytes. (***) Indicates statistical differences observed in relation to control values. See Table 1 . [Ca2+]d, diastolic calcium concentration; FC, functional class; Vm, membrane potential.
These results show that a partial depolarization of the cell membrane occurs in cardiomyocytes from patients with chagasic cardiomyopathy, along with an increase in [Ca2+]d, which is correlated with the level of functional deterioration of the cardiac muscle, according to NYHA classification. We observed a 7% reduction in average Vm values in cardiomyocytes from FCI patients, and a 120% increase in [Ca2+]d. In cells from FCII patients, the mean decrease in Vm was 13% and the increase in [Ca2+]d was 246%. Finally, in cardiomyocytes from FCIII patients we observed a 24% reduction in mean Vm and a 730% increase in [Ca2+]d.
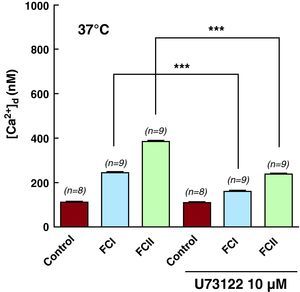
Effect of U-73122 on Intracellular Systolic ConcentrationsTo study the role of inositol 1,4,5-trisphosphate in the dysfunction of intracellular calcium homeostasis of chagasic cardiomyocytes, the cells were treated with 1-[6-[[(17ß)-3-metoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione (U-73122), a β-phospholipase C antagonist involved in the transduction of signals across the plasma membrane, catalyzing the hydrolysis of phospholipids in the membrane from phosphatidylinositol-4,5-biphosphate to inositol 1,4,5-trisphosphate and 1,2-diacylglycerol. Incubation of control cardiomyocytes with 2μM U-73122 for 10min did not reduce [Ca2+]d (112±3.9nM (n=8) before and 109±4nM (n=8) after incubation). However, this same protocol significantly reduced [Ca2+]d in cardiomyocytes taken from FCI patients, from 243±19nM (n=9) to 160±7.5nM (n=9), a decrease of 34%. In cells taken from FCII patients, [Ca2+]d decreased from 383±14nM (n=9) to 238±15nM (n=9), a 38% reduction (Table 2 and Figure 4). We observed no effects on Vm levels. Incubation of cardiomyocytes from FCI and FCII patients with U-7334, an inactive analog, produced no changes in the values of Vm and [Ca2+]d (results not shown). We have no data on the effects of U-73122 on [Ca2+]d in cardiomyocytes from functional class III patients due to the lack of viable cells.
Table 2. Number of Cardiomyocytes Obtained per Patient for the Measurements of Membrane Potential and [Ca2+]d Performed in Different Pharmacological Treatments.
| Patients | Pharmacological treatment | 1 | 2 | 3 | 4 | 5 | 6 |
| FCI | U-73122 | 3 | 2 | 4 | 0 | 0 | 0 |
| 2-APB | 3 | 2 | 3 | 3 | 0 | 0 | |
| Phenylephrine | 4 | 2 | 3 | 0 | 0 | 0 | |
| FCII | U-73122 | 2 | 3 | 2 | 2 | 0 | 0 |
| 2-APB | 0 | 0 | 2 | 2 | 3 | 3 | |
| Phenylephrine | 3 | 2 | 2 | 0 | 2 | 0 |
2-APB, 2-aminoethoxydiphenyl-borate; [Ca2+]d, diastolic calcium concentration; FC, functional class; U-73122, 1-[6-[[(17ß)-3-metoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione.
Does not include FCIII due to the inability to obtain viable cardiomyocytes for the experiment.
Figure 4. Effects of U-73122 on [Ca2+]d in cardiomyocytes obtained from control (healthy subjects), functional class I, and functional class II groups. (***) Indicates statistical differences observed in each group between values taken before and after treatment with U-73122, a ß-phospholipase antagonist. See Table 2 . [Ca2+]d, diastolic calcium concentration; FC, functional class; U-73122, 1-[6-[[(17ß)-3-metoxyestra-1,3,5(10)-trien-17-yl]amino]hexyl]-1H-pyrrole-2,5-dione.
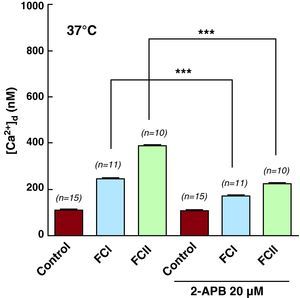
Effects of 2-aminoethoxydiphenyl-borate on Intracellular Diastolic ConcentrationsWe also treated both control and chagasic cardiomyocytes with 2-aminoethoxydiphenyl-borate (2-APB), a membrane-permeant compound that has been proposed as an inhibitor of inositol 1,4,5-trisphosphate receptors. Incubation of control cardiomyocytes with 20mM of 2-APB for 10min had no effect on [Ca2+]d (111±3nM, n=15 before, and 109±5nM, n=15 after treatment with 2-APB), or on Vm (82±2.8mV, n=15 and 84±3.1mV, n=15). However, this same treatment did produce a significant reduction in [Ca2+]d in cardiomyocytes from chagasic patients (Table 2 and Figure 5), although with no modifications to Vm. In chagasic cardiomyocytes from functional class I patients, [Ca2+]d decreased from 245±24nM (n=11) to 172±8nM (n=11), a reduction of 30%. In cells from functional class II patients, [Ca2+]d went from 388±18nM (n=10) to 224±10.7nM (n=10), a 42% reduction. However, we observed no effect of 2-APB on Vm values in chagasic cells obtained from functional class I and II patients (78±2.6mV (n=11) and 71±4.4mV (n=11), respectively). It was not possible to study the effect of 2-APB on [Ca2+]d in cardiomyocytes from functional class III patients due to the lack of viable cells.
Figure 5. Effects of 2-APB on [Ca2+]d in cardiomyocytes from control (healthy subjects), functional class I, and functional class II groups. (***) Indicates statistical differences observed in each group between values before and after treatment with 2-APB. See Table 2 . [Ca2+]d, diastolic calcium concentration; 2-APB, 2-aminoethoxydiphenyl-borate; FC, functional class.
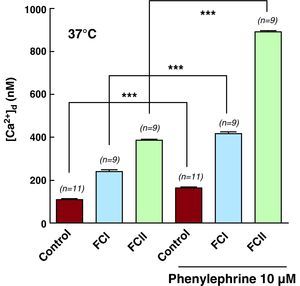
Effects of Phenylephrine on Intracellular Systolic ConcentrationsThe stimulation of α1-adrenergic receptors with phenylephrine is known to activate protein kinase C through a cascade reaction involving a G protein and ß-phospholipase C, which finally produces inositol 1,4,5-trisphosphate and diacylglycerol. Incubation of control and chagasic cardiomyocytes in 10μM of phenylephrine for 5min produced elevated [Ca2+]d in all cells analyzed. However, this increase in [Ca2+]d was greater in chagasic cardiomyocytes than in control cells (FCII>FCI>controls). Phenylephrine raised [Ca2+]d from 110±2.5nM (n=11) to 163±7.7 (n=11) in control cardiomyocytes, from 242±27.8nM (n=9) to 416±21.7nM (n=9) in FCI cardiomyocytes, and from 387±17.4nM (n=9) to 888±20.9nM (n=9) in FCII cardiomyocytes (Table 2 and Figure 6). We did not observe any changes in Vm in any of the cells from the different study groups.
Figure 6. Effects of phenylephrine on [Ca2+]d in cardiomyocytes isolated from control (healthy subjects), functional class I, and functional class II groups. (***) Indicates statistical differences observed in each group between values before and after treatment with phenylephrine, an alpha1-adrenergic receptor agonist. See Table 2 . [Ca2+]d, diastolic calcium concentration; FC, functional class.
The pharmacological effect of phenylephrine on [Ca2+]d in control and chagasic cardiomyocytes was effectively blocked by applying 20μM 2-APB. These results reinforce the hypothesis that altered metabolism of inositol 1,4,5-trisphosphate is associated with chagasic cardiomyopathy. It was not possible to study the effects of phenylephrine on [Ca2+]d in cardiomyocytes taken from functional class III patients due to lack of viable cells.
DiscussionThis study shows a) that ventricular cardiomyocytes from resting patients with chagasic cardiomyopathy experience partial depolarization of the plasma membrane, associated with elevated [Ca2+]d; b) that alterations in Vm and [Ca2+]d are correlated with the level of functional deterioration of the cardiac muscle, established using a clinical evaluation (NYHA classification); c) that incubation of cardiomyocytes in U-73122, a ß-phospholipase C antagonist, or 2-APB, an inhibitor of inositol 1,4,5-trisphosphate receptors, significantly reduced [Ca2+]d values in chagasic but not in control cells; and d) that phenylephrine provoked an increase in [Ca2+]d in chagasic and nonchagasic cardiomyocytes, although this effect was greater in chagasic cells (FCII>FCI>controls).
These results show for the first time that, at the cellular level, chagasic cardiomyopathy is associated with partial depolarization of the cell membrane and increased [Ca2+]d, and that the extent varies according to the clinical state of the patient. We believe that these changes are due solely to the chagasic cardiomyopathy and not secondary side effects from pharmacological treatment, since all medications were suspended 48h before the biopsy to avoid the potential effects of these drugs on the parameters evaluated (Vm and [Ca2+]d). However, it is well known that total elimination of amiodarone from the body is a slow process,7 and the presence of residual amounts of the drug could alter the values of Vm and [Ca2+]d in the cardiomyocytes of chagasic patients who were under treatment. Quian et al.8 reported that amiodarone at clinical concentrations reduces [Ca2+]d values, which is why we believe that the results presented in our study reflect changes induced by the parasitosis rather than from residual pharmacological effects. In the worst case, our measurements of [Ca2+]d would reflect underestimations of the true values, which we can rule out because we found no significant differences between the [Ca2+]d values recorded in cardiomyocytes from chagasic patients who had taken amiodarone and those who had not.
Furthermore, we found strong correlations between the clinical condition of the patient, based on the NYHA classification system, and the functional abnormalities at the cellular level (Vm and [Ca2+]d) produced by the parasitemia. We observed a moderate change in Vm (6% depolarization) and a significant (123%) increase in [Ca2+]d in patients with fewer clinical manifestations (FCI). The magnitude of these alterations was greater in cardiomyocytes from FCII patients, with a depolarization of 10% and increases in [Ca2+]d of 295%, and even more exaggerated results were observed in cells from FCIII patients (depolarization of 22% and [Ca2+]d increase of 738%). Unfortunately, we were unable to study the changes in [Ca2+]d in cardiomyocytes obtained from FCIII individuals in greater detail due to the fragility of the plasma membranes in these cells, as well as the increased amount of connective tissue present, which hindered the separation of viable cardiomyocytes for experimentation (Table 2). In spite of the increased values of [Ca2+]d observed in cardiomyocytes obtained from FCIII cells (with stable Vm), we observed no mechanical activation. One possible explanation is that the concentration of intracellular Ca2+ (a physiological regulator of muscle contraction) did not reach critical levels (1μM), which would inhibit the effects of troponin C, allowing the actin-myosin interaction. However, other studies have demonstrated mechanical activity in striated muscle fibers at lower intracellular Ca2+ concentrations (0.5μM). These differences could be explained by physiological differences between animal models, or by the different cell preparations used in each experiment (naked fibers –all plasma membrane removed– or fibers with a hyperpermeable plasma membrane). The differences could also be affected by several other factors, such as: a) the Ca2+ load of the SR9; b) the [Mg2+] in the area of the contractile microfilaments; c) temperature, which affects the processes of release and uptake of Ca2+ by the SR9 and modifies the sensitivity of myofilaments to Ca2+ ions; and d) the initial length of the sarcomere, which determines the magnitude of the voltage generated in both skeletal and cardiac muscle.
Changes in the [Ca2+]d found in chagasic cells are qualitatively similar to those reported in epithelial cells infected with T. cruzi, in which a significant increase can be appreciated in the baseline values of intracellular [Ca2+], as well as altered patterns of Ca2+ movement in response to bradykinin and other agonists.10 The [Ca2+]d in cardiac muscle depends on a series of physiological mechanisms located in the plasma membrane (Na+/Ca2+ exchanger and Ca2+-ATPase pump) and the sarcoplasmic reticulum (the Ca2+-ATPase pump).11 Normal functioning of these mechanisms allows for maintaining proper [Ca2+]d during the rest period of the cardiac cycle (diastole) within a physiological range (∼100nM).12 The fact that [Ca2+]d in chagasic cardiomyocytes reaches abnormal values implies a loss of intracellular regulation of this cation. This could be associated with poor functioning of one or several of these intracellular calcium homeostasis related mechanisms, induced by the parasite either directly or indirectly. Likewise, this could be a direct consequence of a prolonged increase in calcium release from the sarcoplasmic reticulum or uptake from the exterior, mediated by the intracellular messenger inositol 1,4,5-trisphosphate, in response to adrenergic overstimulation or activation of the inositol 1,4,5-trisphosphate metabolism, whether induced directly or indirectly by the parasite.
Inositol 1,4,5-trisphosphate, an intracellular messenger present in striated (cardiac and skeletal) and smooth muscle, is capable of releasing calcium from intracellular deposits by activating receptors (calcium channels) located in the membrane of the sarcoplasmic reticulum or regulating the entry of calcium ions into the cell from the extracellular space.13 Type 1 and 2 inositol 1,4,5-trisphosphate receptors have been identified in several areas of cardiac muscle. Whereas type 1 inositol 1,4,5-trisphosphate predominates in cardiac neural cells, type 2 is primarily located in cardiomyocytes located along Z-lines, in the perinuclear region, and in the nuclear membrane.14 However, the functional relevance of the different types of inositol 1,4,5-trisphosphate receptors in the heart remains controversial.15 Several publications have suggested that among the functions of these receptors may be the regulation of genetic transcription,15 the amplification of ryanodine receptor signals,16 and the regulation of calcium uptake from the extracellular space.15 Other studies suggest that they may be involved in the pathophysiology of ventricular hypertrophy, atrial fibrillation, ventricular tachycardia, and cardiac hypoxia.
Our results indicate that the increase in [Ca2+]d observed in chagasic cardiomyocytes is partially mediated by inositol 1,4,5-trisphosphate, since treatment with U-73122, a ß-phospholipase C inhibitor, and 2-APB, an inositol 1,4,5-trisphosphate receptor blocker, partially reduced the elevated [Ca2+]d in the chagasic cardiomyocytes from functional group I and II patients. This reduction in [Ca2+]d was greater in chagasic cardiomyocytes from functional class II (38% and 41% when induced by U-73122 and 2-APB, respectively) than in those obtained from chagasic patients in functional class I (34% and 29%, respectively). This increase in [Ca2+]d seems to be associated with increased metabolism of phosphoinositides, which leads to greater calcium release from the SR and/or an increase in calcium uptake from the extracellular space during diastole. This potential phosphoinositide metabolism dysfunction varies according to the clinical state of the patients, as the abnormalities were more evident in cardiomyocytes from patients in FCII than in those from FCI. This hypothesis was reinforced by the greater effect of phenylephrine on [Ca2+]d in chagasic cardiomyocytes (1.6 times and 2.3 times in FCI and FCII, respectively) than in controls (1.4 times), and due to the fact that the effect of phenylephrine on [Ca2+]d was inhibited by 2-APB, a blocker of inositol 1,4,5-trisphosphate (type 3) in all cell groups.
Previous studies have demonstrated the presence of M3-muscarinic receptors in ventricular cells of the human heart.17 Overexpression of these receptors has been observed in the hearts of animals infected with T cruzi.18 Additionally, these M3-muscarinic receptors are preferentially coupled to Gq/11proteins, which mediate the activation of phospholipase C, which, once activated, produces the hydrolysis of phosphatidylinositol 4,5-biphosphate to produce inositol 1,4,5-trisphosphate and diacylglycerol.19
ConclusionsThe evidence presented allows us to obtain a greater comprehension of the pathophysiology of chagasic cardiomyopathy, specifically with regard to the participation of inositol 1,4,5-trisphosphate in the dysfunctional regulation of [Ca2+]d in chagasic cardiomyocytes. Our results do not completely explain this alteration in intracellular calcium homeostasis, since although U-73122 and 2-APB did significantly reduce [Ca2+]d, they did not produce complete normalization to physiological levels. Additionally, none of the pharmacological treatments used were able to revert the partial depolarization of the cell membrane produced in chagasic cells. This would suggest the presence of other possible abnormalities induced directly or indirectly by the parasite. These may be present in other structures or metabolic pathways, such as the dihydropyridine receptor in the plasma membrane (slow Ca2+ channel) or the ryanodine receptor (Ca2+channel) in the sarcoplasmic reticulum. New studies will be necessary to deduce the complete pathophysiology of this condition.
Conflicts of interestNone declared.
Acknowledgements
We would like to thank Drs. Carlos Moratinos and Michael Ferguson for providing the endomyocardial biopsies.
Received 27 April 2010
Accepted 21 January 2011
Corresponding author: Apartado de correo 2127, Centro de Biofísica y Bioquímica, Instituto Venezolano de Investigaciones Científicas, Caracas 1020A, Venezuela. amijares@ivic.gob.ve