There is uncertainty on the correct management of antithrombotic therapies after transcatheter aortic valve replacement (TAVR), with dual antiplatelet therapy (DAPT) being currently recommended on an empirical basis. The aim of the present meta-analysis was to assess the safety and effectiveness of DAPT in patients undergoing TAVR.
MethodsStudies comparing different antithrombotic regimens after TAVR were included. The primary endpoint was 30-day overall mortality.
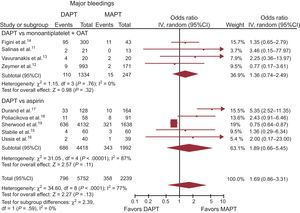
ResultsWe included 9 studies, 5 comparing DAPT with aspirin monotherapy and 4 comparing DAPT with monoantiplatelet therapy (MAPT) + oral anticoagulation. Among 7991 patients, 72% were on DAPT. The median follow-up was 3.5 months. Mortality was significantly lower in the DAPT group (12.2% vs 14.4%; OR, 0.81; 95%CI, 0.70-0.93; P = .003; Phet = .93), with similar benefits compared with aspirin monotherapy (OR, 0.80; 95%CI, 0.69-0.93; P = .004; Phet = .60), which were not statistically significant when compared with MAPT + oral anticoagulation (OR, 0.86; 95%CI, 0.55-1.35; P = .51; Phet = .97). A similar trend for DAPT was observed for stroke (OR, 0.83 95%CI, 0.63-1.10; P = .20; Phet = .67), with no increase in the rate of major bleedings (OR, 1.69; 95%CI, 0.86-3.31; P = .13; Phet< .0001). On indirect comparison analysis, no benefit in survival, stroke, or bleedings was identified for additional oral anticoagulation.
ConclusionsThe present meta-analysis supports the use of DAPT after TAVR, reducing mortality and offering slight benefits in stroke, with no increase in major bleedings compared with MAPT. The strategy of aspirin + oral anticoagulation did not provide significant benefits compared with MAPT or DAPT.
Keywords
Transcatheter aortic valve replacement (TAVR) is becoming a main option for the treatment of patients with severe aortic valve stenosis, especially for those higher-risk subsets of patients that are not amenable to surgical valve replacement.1,2
However, despite the technological improvements, the risk of periprocedural or long-term complications is still relevant, including the occurrence of hemorrhagic events in up to 41% of transcatheter aortic valve implantation (TAVI) procedures,3 mainly due to access-site bleedings, while stroke affects 6% of patients.4
Current guidelines, therefore, recommend dual antiplatelet therapy (DAPT) combining low-dose aspirin and a thienopyridine early after TAVI and for up to 6 months.5 Nevertheless, evidence supporting these indications is still weak and recent meta-analyses have even shown an increased risk of bleeding complications with more potent antiplatelet strategies.6,7
Indeed, the balance between hemorrhagic and thrombotic risk is challenging among the elderly and frail patients who are usually candidate for TAVR. Moreover, a potential beneficial role of short-term anticoagulation has been proposed in order to improve stroke prevention, in accordance with the strategy applied for surgical aortic valve replacement, and mainly in patients with concomitant atrial fibrillation.8
Ongoing randomized trials are attempting to identify the ideal antithrombotic strategy after TAVI procedures. However, until new data are available, therapeutic indications on the safety and effectiveness of DAPT or anticoagulation can be only provided from meta-analyses of available studies, which was, therefore, the aim of our study.
METHODSEligibility and Search StrategyThe literature was scanned by formal searches of electronic databases (MEDLINE, Cochrane and EMBASE) for clinical studies and from scientific session abstracts, searched on the Transcatheter Cardiovascular Therapeutics, EuroPCR, American College of Cardiology, American Heart Association, and European Society Cardiology websites, for oral presentations and/or expert slide presentations from January 1990 to December 2015.
Studies were included if they compared a dual antiplatelet strategy with monoantiplatelet therapy (MAPT), with or without oral anticoagulation, (OAC) after TAVI.
The following keywords were used: “antiplatelet”, “dual antiplatelet therapy”; “anticoagulation”, “transcatheter aortic valve implantation”; “TAVI”.
No language restrictions were enforced. Inclusion criteria were: a) patients undergoing TAVI, b) availability of complete clinical data, and c) different antithrombotic treatment allocation. Exclusion criteria were: a) follow-up data in less than 90% of patients, b) ongoing studies or irretrievable data, and c) use of triple antithrombotic therapy (DAPT + OAC).
Data Extraction and Validity AssessmentData were independently abstracted by 2 investigators (M. Verdoia and L. Barbieri). If the data were incomplete or unclear, the authors were contacted. Disagreements were resolved by consensus. Data were managed according to the intention-to-treat principle.
Outcome MeasuresThe primary endpoint was overall mortality for DAPT vs MAPT ±OAC. The secondary endpoint was the occurrence of stroke. The safety endpoint was defined as the occurrence of major bleeding complications (according to protocol definition) with DAPT vs other strategies. Adjusted indirect comparison for MAPT plus OAC therapy vs MAPT alone was then performed for the 3 different study endpoints.
Data AnalysisStatistical analysis was performed using the Review Manager 5.3 freeware package. Odds ratios (OR) and 95% confidence intervals (95%CI) were used as summary statistics. The pooled OR was calculated by using a fixed or random effect model (DerSimonian and Laird random-effects model, if there was significant heterogeneity among studies). The Breslow-Day test was used to examine the statistical evidence of heterogeneity across the studies (P < .1).
Study quality was evaluated by the same 2 investigators according to a score, that, as previously described,9 was expressed on an ordinal scale, allocating 1 point for the presence of each of the following: a) statement of objectives, b) explicit inclusion and exclusion criteria, c) description of intervention, d) objective means of follow-up, e) description of adverse events, f) power analysis, g) description of statistical methods, h) multicenter design, i) discussion of withdrawals, and j) randomized design.
A meta-regression analysis was carried out to evaluate the relationship between benefits in mortality from DAPT vs MAPT and patients’ risk profile (as log of the OR for mortality in the control group) or the difference in major bleeding complications.
An adjusted indirect comparison of pooled estimates was then performed according to Biondi-Zoccai et al.9 Specifically, we generated from fixed-effect OR comparing MATP or MAPT + OAC vs DAPT an interaction OR for MAPT vs MAPT + OAC, with pertinent 95%CI and z scores for 2-tailed hypothesis testing (P significant if < .05).
The study was performed in compliance with the PRISMA guidelines.10
RESULTSEligible StudiesA total of 11 studies were identified.11–21 Among them, 2 studies20,21 were excluded for including patients on DAPT + OAC in the control group.
Therefore, 9 studies were finally included, 515–19 comparing DAPT with aspirin monotherapy and 4 studies comparing DAPT with MAPT + OAC.11–14 The flowchart for the process of selecting studies is displayed in Figure 1.
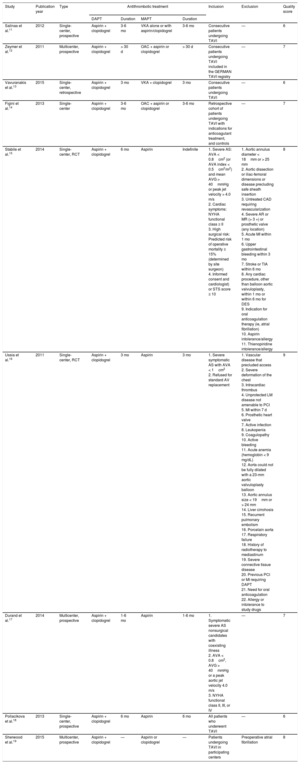
In a total population of 7991 included patients, 5752 (72%) were on DAPT. In 5% of patients receiving a single antiplatelet agent, OAC was associated, mainly for clinical indications (either pre-existing conditions, ie, mechanical valve prosthesis, previous thrombotic/thromboembolic event or atrial fibrillation). The characteristics of included studies are listed in Table 1, while Table 2 displays the main clinical features of the study populations.
Characteristics of Included Studies
| Study | Publication year | Type | Antithrombotic treatment | Inclusion | Exclusion | Quality score | |||
|---|---|---|---|---|---|---|---|---|---|
| DAPT | Duration | MAPT | Duration | ||||||
| Salinas et al.11 | 2012 | Single-center, prospective | Aspirin + clopidogrel | 3-6 mo | VKA alone or with aspirin/clopidogrel | 3-6 mo | Consecutive patients undergoing TAVI | — | 6 |
| Zeymer et al.12 | 2011 | Multicenter, prospective | Aspirin + clopidogrel | > 30 d | OAC + aspirin or clopidogrel | > 30 d | Consecutive patients undergoing TAVI included in the GERMAN TAVI registry | — | 7 |
| Vavuranakis et al.13 | 2015 | Single-center, retrospective | Aspirin + clopidogrel | 3 mo | VKA + clopidogrel | 3 mo | Consecutive patients undergoing TAVI | — | 6 |
| Figini et al.14 | 2013 | Single-center | Aspirin + clopidogrel | 3-6 mo | OAC + aspirin or clopidogrel | 3-6 mo | Retrospective cohort of patients undergoing TAVI with indications for anticoagulant treatment, and controls | — | 7 |
| Stabile et al.15 | 2014 | Single-center, RCT | Aspirin + clopidogrel | 6 mo | Aspirin | Indefinite | 1. Severe AS: AVA < 0.8cm2 (or AVA index < 0.5cm2/m2) and mean AVG > 40mmHg or peak jet velocity > 4.0 m/s 2. Cardiac symptoms: NYHA functional class ≥ II 3. High surgical risk: Predicted risk of operative mortality ≥ 15% (determined by site surgeon) 4. Informed consent and cardiologist) or STS score ≥ 10 | 1. Aortic annulus diameter < 18mm or > 25 mm 2. Aortic dissection or iliac-femoral dimensions or disease precluding safe sheath insertion 3. Untreated CAD requiring revascularization 4. Severe AR or MR (> 3 +) or prosthetic valve (any location) 5. Acute MI within 1 mo 6. Upper gastrointestinal bleeding within 3 mo 7. Stroke or TIA within 6 mo 8. Any cardiac procedure, other than balloon aortic valvuloplasty, within 1 mo or within 6 mo for DES 9. Indication for oral anticoagulation therapy (ie, atrial fibrillation) 10. Aspirin intolerance/allergy 11. Thienopiridine intolerance/allergy | 8 |
| Ussia et al.16 | 2011 | Single-center, RCT | Aspirin + clopidogrel | 3 mo | Aspirin | 3 mo | 1. Severe symptomatic AS with AVA < 1cm2 2. Refused for standard AV replacement | 1. Vascular disease that precluded access 2. Severe deformation of the chest 3. Intracardiac thrombus 4. Unprotected LM disease not amenable to PCI 5. MI within 7 d 6. Prosthetic heart valve 7. Active infection 8. Leukopenia 9. Coagulopathy 10. Active bleeding 11. Acute anemia (hemoglobin < 9 mg/dL) 12. Aorta could not be fully dilated with a 23-mm aortic valvuloplasty balloon 13. Aortic annulus size < 19mm or > 24 mm 14. Liver cirrohosis 15. Recurrent pulmonary embolism 16. Porcelain aorta 17. Respiratory failure 18. History of radiotherapy to mediastinum 19. Severe connective tissue disease 20. Previous PCI or MI requiring DAPT 21. Need for oral anticoagulation 22. Allergy or intolerance to study drugs | 9 |
| Durand et al.17 | 2014 | Multicenter, prospective | Aspirin + clopidogrel | 1-6 mo | Aspirin | 1-6 mo | 1. Symptomatic severe AS nonsurgical candidates with coexisting illness 2. AVA < 0.8cm2, AVG > 40mmHg or a peak aortic jet velocity 4.0 m/s 3. NYHA functional class II, III, or IV | — | 7 |
| Poliacikova et al.18 | 2013 | Single-center, prospective | Aspirin + clopidogrel | 6 mo | Aspirin | 6 mo | All patients who underwent TAVI | — | 6 |
| Sherwood et al.19 | 2015 | Multicenter, prospective | Aspirin + clopidogrel | — | Aspirin or clopidogrel | — | Patients undergoing TAVI in participating centers | Preoperative atrial fibrillation | 8 |
AR, aortic regurgitation; AS, aortic stenosis; AVA, aortic valve area; AVG, aortic mean gradient; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; DES, drug-eluting stent; LM, left main disease; MAPT, mono-antiplatelet therapy; MI, myocardial infarction; MR, mitral regurgitation; NYHA; New York Heart Association; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; RCT, randomized clinical trial; STS, Society of Thoracic Surgery; TAVI, transcatheter aortic valve implantation; TIA, transient ischemic attack; VKA, vitamin K antagonist.
Clinical Features of Patients in Included Studies
| Study | DAPT, no. | MAPT, no. | Device | Access | Primary endpoint | Bleeding definition | Maximum follow-up | Mean age DAPT, y | Mean age MAPT, y | Women DAPT, % | Women MAPT, % | CAD DAPT, % | CAD MAPT, % | Pre-TAVI AF DAPT, % | Pre-TAVI AF MAPT, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Salinas et al.11 | 21 | 13 | Edwards-Sapien | Transfemoral, transapical | Death, myocardial infarction, stroke, MACE | — | In-hospital | 81.30 | 83.90 | 64.70 | 58.80 | 35.30 | 58.80 | 100.00 | 0.00 |
| Zeymer et al.12 | 993 | 171 | — | — | Death | — | 30 d | — | — | — | — | — | — | — | — |
| Vavuranakis et al.13 | 20 | 20 | CoreValve | Transfemoral, transubclavian | Cardiac death, myocardial infarction, any coronary revascularization, and stroke at follow-up | BARC | Mean 23.4 mo | 80.20 | 80.60 | 60.00 | 60.00 | 35.00 | 60.00 | 100.00 | 0.00 |
| Figini et al.14 | 300 | 43 | Edwards-Sapien or CoreValve | Transfemoral, transapical | Death | — | 11-12 mo | 80.00 | 79.00 | 51.00 | 48.00 | 26.00 | 46.00 | 100.00 | 0.00 |
| Stabile et al.15 | 60 | 60 | Edwards-Sapien | Transfemoral | Death | VARC | 6 mo | 80.20 | 81.10 | 66.70 | 60.00 | 21.30 | 23.30 | 15.60 | |
| Ussia et al.16 | 40 | 39 | CoreValve | Transfemoral, transapical | Death from any cause, MI, major stroke, urgent or emergency conversion to surgery, and life-treatening bleeding | — | 6 mo | 80.00 | 81.00 | 50.00 | 59.00 | — | — | 0.00 | 0.00 |
| Durand et al.17 | 128 | 164 | Edwards-Sapien or CoreValve | Transfemoral. Transsubclavian transapical or transaortic | Mortality, major stroke, life-threatening bleeding, MI, and major vascular complications | — | 30 d | 84.60 | 82.70 | 60.90 | 45.10 | 30.50 | 50.00 | 10.00 | 15.00 |
| Poliacikova et al.18 | 58 | 91 | Edwards-Sapien or CoreValve or Lotus | Transfemoral. Transsubclavian, transapical or transaortic | All-cause mortality, acute coronary event, stroke, or major bleeding | VARC | 30 d | 81.60 | 82.00 | 44.60 | 46.20 | — | — | 35.20 | 23.00 |
| Sherwood et al.19 | 4132 | 1638 | — | — | Death | — | 12 mo | 84.00 | 84.00 | 51.60 | 53.10 | 67.10 | 55.70 | 27.60 | 11.00 |
AF, atrial fibrillation; BARC, Bleeding Academic Research Consortium; CAD, coronary artery disease; DAPT, dual antiplatelet therapy; MACE, major acute cardiovascular events; MAPT, mono-antiplatelet therapy; MI, myocardial infarction; TAVI, transcatheter aortic valve implantation; VARC, Valve Academic Research Consortium.
Transcatheter aortic valve replacement was performed mainly through a transfemoral approach, but 5 studies allowed transapical11,14,16–18 and 2 transaortic access,17,18 whereas a transsubclavian approach was considered in 3 studies.13,17,18
Dual antiplatelet therapy duration ranged from 1 to 6 months in 2 studies,12,18 from 3 to 6 months in other 2,11,14, and while was scheduled for exactly 3 months in 2 studies13,16 and 6 months in 2 studies.15,18 Monoantiplatelet therapy consisted of aspirin in most patients, while clopidogrel alone was allowed in 4 studies, in association with OAC, and in 1 other registry.11–14,19
The median follow-up was 3.5 months. In 1 study, only in-hospital data were collected,11 whereas 3 studies provided outcomes at 30 days12,17,18 and 2 studies at 6 months.15,16 In 3 studies, follow-up was 1 year or longer.13,14,19
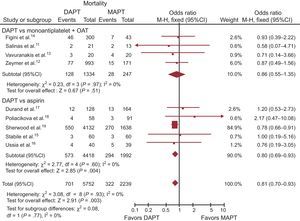
Clinical OutcomePrimary EndpointData on overall mortality were available in 7991 (100%) of the patients. Death occurred in 1023 (12.8%) patients. Mortality was significantly lower in the DAPT group than in the MAPT group (OR, 0.81; 95%CI, 0.70-0.93; P = .003; Phet = .93), as displayed in Table 3 and Figure 2. Similar benefits were observed with aspirin monotherapy (OR, 0.80; 95%CI, 0.69-0.93; P = .004, Phet = .60), while not reaching statistical significance when compared with MAPT + OAC (OR, 0.86; 95%CI, 0.55-1.35; P = .51, phet = .97).
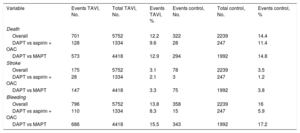
Total and Percentage of Events for Primary and Secondary Study Endpoints in the Transcatheter Aortic Valve Implantation and Control Group
| Variable | Events TAVI, No. | Total TAVI, No. | Events TAVI, % | Events control, No. | Total control, No. | Events control, % |
|---|---|---|---|---|---|---|
| Death | ||||||
| Overall | 701 | 5752 | 12.2 | 322 | 2239 | 14.4 |
| DAPT vs aspirin + OAC | 128 | 1334 | 9.6 | 28 | 247 | 11.4 |
| DAPT vs MAPT | 573 | 4418 | 12.9 | 294 | 1992 | 14.8 |
| Stroke | ||||||
| Overall | 175 | 5752 | 3.1 | 78 | 2239 | 3.5 |
| DAPT vs aspirin + OAC | 28 | 1334 | 2.1 | 3 | 247 | 1.2 |
| DAPT vs MAPT | 147 | 4418 | 3.3 | 75 | 1992 | 3.8 |
| Bleeding | ||||||
| Overall | 796 | 5752 | 13.8 | 358 | 2239 | 16 |
| DAPT vs aspirin + OAC | 110 | 1334 | 8.3 | 15 | 247 | 5.9 |
| DAPT vs MAPT | 686 | 4418 | 15.5 | 343 | 1992 | 17.2 |
DAPT, dual antiplatelet therapy; MAPT, monoantiplatelet therapy; OAC, oral anticoagulation; TAVI, transcatheter aortic valve implantation.
Dual antiplatelet therapy vs MAPT with or without OAT overall mortality with OR and 95%CI. The size of the data markers (squares) for aspirin is approximately proportional to the statistical weight of each trial. 95%CI, 95% confidence interval; DAPT, dual antiplatelet therapy; MAPT, monoantiplatelet therapy; M-H, Mantel-Haenszel; OAT, oral anticoagulation therapy; OR, odds ratio.
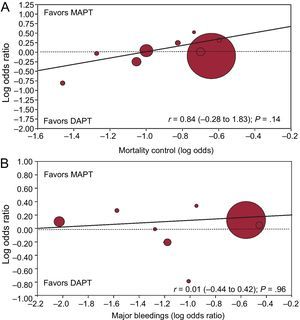
Meta-regression analysis showed no association between the survival benefits of DAPT (as log OR for mortality) and patients’ risk profile (defined as log OR for mortality in the control group; r = 0.84; 95%CI, −0.28 to 1.83; P = .14) and the risk of major bleedings with DAPT vs MAPT (as log OR for major bleedings; r = 0.01; 95%CI, −0.44 to 0.42; P = .96), as displayed in Figure 3.
Random effect meta-regression analyses for the risk (OR) of mortality between DAPT and MAPT according to patients’ risk profile (A) or the differential risk of bleedings in the 2 arms (B). The size of the circle corresponds to the statistical weight of each study. DAPT, dual antiplatelet therapy; MAPT, monoantiplatelet therapy; OR, odds ratio.
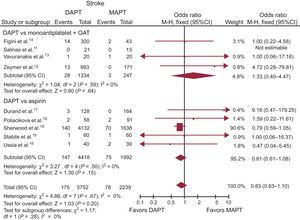
Stroke. Data on stroke were available in 100% of the study population (7991 patients). Stroke occurred in 253 (3.2%) patients, with a slightly non-significant lower rate in patients on DAPT (OR, 0.83; 95%CI, 0.63-1.10; P = .20; Phet = .67) (Table 3 and Figure 4). No significant difference in stroke was observed between DAPT vs MAPT (OR, 0.81; 95%CI, 0.61-1.08; P = .15; Phet = .50), and MAPT + OAC (OR, 1.33; 95%CI, 0.40-4.47; P = .64; Phet = .59).
Dual antiplatelet therapy vs MAPT with or without OAT on stroke with OR and 95%CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. 95%CI, 95% confidence interval; DAPT, dual antiplatelet therapy; MAPT, monoantiplatelet therapy; M-H, Mantel-Haenszel; OAT, oral anticoagulation therapy; OR, odds ratio.
Meta-regression analysis showed no association between the reduction in the rate of stroke with DAPT (as log OR for stroke) and patients’ risk profile (defined as log OR for stroke in the control group; r = −2.22; 95%CI (−5.8 to 1.29); P = .21).
Major Bleedings. Among the 7991 patients whose data were available, a major bleeding complication, as per protocol definition, occurred in 14.4% (1154) patients.
A more aggressive dual antiplatelet strategy was not associated with an increased risk of major bleedings (OR, 1.69; 95%CI, 0.86-3.31; P = .13; Phet< .0001), as displayed in Table 3 and Figure 5. Similar results were obtained for DAPT vs MAPT (15.5% [686/4418] vs 17.2% [343/1992], OR, 1.89; 95%CI, 0.66-5.45; P = .11; Phet< .00001), and MAPT + OAC (OR, 1.36; 95%CI, 0.74-2.49; P = .32; Phet = .76).
Dual antiplatelet therapy vs MAPT with or without OAT on major bleedings with OR and 95%CI. The size of the data markers (squares) is approximately proportional to the statistical weight of each trial. 95%CI, 95% confidence interval; DAPT, dual antiplatelet therapy; MAPT, monoantiplatelet therapy; OAT, oral anticoagulation therapy; OR, odds ratio.
Meta-regression analysis showed no association between the reduction in the rate of major bleedings with DAPT (as log OR) and patients’ risk profile (defined as log OR for major bleedings in the control group; r = −0.21; 95%CI, (−1.59 to 1.16), P = .76).
Adjusted Indirect Comparison. Head-to-head comparison of MAPT + OAC vs MAPT alone showed no difference in mortality (OR, 0.93; 95%CI, 0.58-1.51; z, 0.27; P = .78); stroke (OR, 0.51; 95%CI, 0.15-1.72; z, 1.08; P = .28), or major bleedings (OR, 0.61; 95%CI, 0.31-1.14; z, 1.54; P = .12) with the 2 different antithrombotic strategies.
DISCUSSIONThe present meta-analysis represents the most comprehensive study addressing the impact of antithrombotic strategies on clinical outcomes in patients undergoing TAVR. Our main finding was a significant reduction in mortality with no impact on major bleedings with DAPT compared with MAPT, even when the single antiplatelet agent was associated with anticoagulation, thus supporting the strategy currently suggested by guidelines and major expert consensus.
Transcatheter aortic valve replacement represents an innovative strategy for the management of patients with severe aortic valve stenosis, who are deemed unsuitable for surgical valve replacement.22 Technological improvements have allowed the achievement of results comparable to traditional surgical replacement and a reduction in the rate of major procedural complications, mainly by reducing the rate of paravalvular leakage and access-site invasivity.23,24
Nevertheless, both ischemic and hemorrhagic complications are still not irrelevant, especially in a frail, comorbidity-rich subset of patients such as those undergoing TAVR, indicating the importance of the pivotal role of antithrombotic therapies.25
However, uncertainty still exists on the most appropriate antithrombotic strategy to be administered after valve implantation. While short-term OAC could be expected to be the best option, in accordance with the indications for surgical aortic valve replacement, DAPT has emerged from the outset as the preferred approach, mimicking the strategy applied for percutaneous coronary stent implantation without TAVI.26
The 2014 American Heart Association and American College of Cardiology Guidelines currently recommend DAPT consisting of clopidogrel and aspirin for 6 months.5 Similarly, the Canadian Cardiovascular Society recommends the use of aspirin indefinitely, and a combination with clopidogrel for 1 to 3 months, and analog indications are provided by the European Society of Cardiology.1,26 Nevertheless, these recommendations are based on the results of the first TAVI trial, the PARTNER trial, in which patients randomized to TAVR received DAPT,2 although few studies have so far compared different antithrombotic therapies after TAVR.
Two small randomized trials have been conducted so far, comparing DAPT with aspirin monotherapy,15,16 and showing no difference in clinical outcomes between the 2 different strategies, although the addition of clopidogrel was associated with a modest increase in the rate of bleedings. Similar results were then confirmed in 2 retrospective studies17,18 and subsequent meta-analyses,7,27 suggesting that a treatment with aspirin could be justified against DAPT, offering similar survival benefits and a lower hemorrhagic risk.
The opposite tendency to enhance antithrombotic treatment, nevertheless, should be advocated when considering the risk of cerebrovascular ischemia after TAVI. In fact, although more than 50% of these events occur in the periprocedural phase, due to valve calcium embolization or the manipulation of catheters into an atheromasic aorta, an increased risk of stroke remains for up to 2 months after the procedure; this risk has been reported due to thromboembolism.28 The proposed mechanisms include a prothrombotic state of the valve leaflets prior to their complete endothelialization within the first 3 months, as well as atrial fibrillation.25,29 The latter, in fact, has been observed in up to 40% of TAVI patients, with a relevant impact on mortality and a potential larger effect on the role of long-term antithrombotic strategies.11,30 Indeed, the cessation of anticoagulation has been shown to reduce the survival of patients with atrial fibrillation31; in contrast, no clear benefit has emerged when associating OAC with antiplatelet agents.32 Moreover, the association of antiplatelet agents with OAC has to be weighed against an increased risk of bleedings,33,34 with no indication being provided, so far, on the optimal combination of platelet inhibitors in patients requiring anticoagulation after TAVR.35
In addition, the recently presented US STS/ACC TVT Registry19 has clearly shown in a huge population of TAVR patients that DAPT could improve survival and was even associated with a lower rate of bleeding complications.
Thus, in a field of uncertainty and with a lack of dedicated studies, the aim of the present meta-analysis was to provide data on the safety and effectiveness of DAPT vs MAPT, with or without anticoagulation, in patients undergoing TAVR.
We included a large population of about 8000 patients, including both randomized trials and registries. We included more than 5000 patients on DAPT, who displayed a lower mortality and a modest reduction in cerebrovascular events compared with MAPT. Moreover, the benefits of DAPT were not affected by bleeding complications.
The association of OAC with MAPT was not only inferior to DAPT in lowering mortality, but did not offer any significant advantage in comparison with MAPT alone. However, the nonrandomized design of most studies might have led to the inclusion in the MAPT arm of more frail and critically-ill patients who were deemed at higher bleeding risk and who also had a higher risk of mortality. In addition, we could not evaluate the prevalence of other comorbidities and especially the rate of patients with concomitant coronary artery disease treated with stenting who might have derived the greatest benefits from DAPT.
In our study, OAC-treated patients represented only a small part of the overall population, although potentially displaying a different ischemic and hemorrhagic risk compared with patients treated with antiplatelet agents. However, our subgroup analysis comparing DAPT with MATP + OAC provided analog results for the subgroup of DAPT compared with MAPT alone. Indeed, we could not stratify these patients according to the indication for OAC, thus potentially including both patients with atrial fibrillation; stroke or high thrombotic risk and patients treated in centers where OAC is commonly used after TAVI, potentially providing an explanation for our comparable results. In fact, the outcomes benefits observed with DAPT were consistent across the entire study population and were not influenced by patients’ risk profile.
Thus, the present findings actually support the current recommendations of using DAPT as the best antithrombotic strategy in patients undergoing TAVR, whereas the association of OAC with antiplatelet therapy should be carefully balanced and limited to patients with strict preprocedural indications for OAC, such as the presence of mechanical prosthetic valves or chronic atrial fibrillation, offering no advantage in comparison with DAPT.
Nevertheless, the ongoing larger randomized trials36–38 will certainly provide clearer evidence on this topic and offer indications for the optimal duration of DAPT after TAVR.
LimitationsCertain limitations should be addressed in the present study, of which the most important relates to the synthesis of data from different trials. In particular, the inclusion of nonrandomized studies led to a lack of proportion between the arm on DAPT and patients receiving single antiplatelet therapy, and especially for those requiring an association with OAC, who represented only a minority of our study population. However, the present positive findings for DAPT further support the strategy currently in use in most real-life patients.
In addition, most studies were limited by the small sample size, with meta-analysis results being driven mainly by the huge US STS/ACC TVT Registry.19 Moreover, the definition of mortality differed among the studies (overall mortality, cardiovascular mortality, in-hospital mortality). However, no significant heterogeneity was found for our primary endpoint and, furthermore, due to the modest number of available data we preferred to include all available studies in order to avoid a potential selection bias.
Another limitation consisted in the differences in DAPT duration or in the follow-up period, with few data being reported for long-term periods. Nevertheless, it might be expected that the greatest differences in bleedings and in the prevention of thromboembolic events could be observed during the periprocedural treatment period. In addition, the availability of data at long-term follow-up would have allowed better identification of the benefits of the antithrombotic therapies in preventing cardiovascular events. However, we preferred not to exclude studies reporting only in-hospital data, since this would have led to the exclusion of those more critically-ill patients, who experienced early mortality.
Finally, most of these studies were conducted with first generations of valve prosthesis and different results could be expected with the introduction of new devices that have dramatically lowered the rate of access-site hemorrhagic complications and the rate of cerebrovascular ischemic events.
CONCLUSIONSThe present meta-analysis provides evidence for the current recommendation of DAPT as the preferred antithrombotic strategy in patients undergoing TAVR. In fact, DAPT provided a significant reduction in mortality and a slight benefit in stroke, with no increase in major bleedings as compared with MAPT. The strategy of aspirin and anticoagulation did not provide significant benefits compared with MAPT or DAPT.
CONFLICTS OF INTERESTNone declared.
- –
Antithrombotic treatment in patients undergoing TAVR is still debated, with few data being derived from randomized trials.
- –
Dual antiplatelet therapy is currently recommended on an empirical basis, while the potential role of adjunctive anticoagulation is unclear.
- –
We performed a meta-analysis of 9 trials comparing DAPT with aspirin monotherapy with or without OAC.
- –
Dual antiplatelet therapy in TAVR patients reduced mortality and offered slight benefits in stroke, with no increase in major bleedings.
- –
Adjunctive anticoagulation did not provide any significant benefits.