Keywords
INTRODUCTION
The pericardium is a sac comprising 2 fibroserous layers that surrounds the heart. The pericardium can be affected by a wide variety of disease-causing agents and processes ranging from viral and bacterial infections and infections with other microorganisms to invasion by neoplastic diseases. The pericardium can also be affected by nonspecific inflammatory processes or conditions secondary to heart disease or systemic disease.1-3 However, most pericardial diseases are caused by a limited number of relatively common processes that are generally easy to identify. The repercussions of these different etiologies can be classified into 3 basic types of clinical manifestations. The first type corresponds to symptoms derived from pericardial inflammation that essentially present as pericardial pain and fever, the second to symptoms due to pericardial effusion which, in its most severe form, may lead to cardiac tamponade, and the third to symptoms due to thickening, retraction, and calcification of the pericardium, indicative of constrictive pericarditis.
The basic aim of this article is to describe diagnosis and treatment of acute pericarditis. We will also comment on some aspects of cardiac tamponade and its treatment, given that this complication can present with any type of pericarditis. A review of constrictive pericarditis is not within the scope of this article, but we will discuss some forms of acute and subacute cardiac constriction that may present early in the course of some acute pericarditis.
ACUTE PERICARDITIS
Acute pericarditis is a clinical syndrome with many possible causes that presents with chest pain, pericardial friction rub, and gradual repolarization changes in the electrocardiogram (ECG). Diagnosis of pericarditis requires at least 2 of these 3 elements, although auscultation of pericardial friction rub can be sufficient for diagnosis on its own. Many different causes of pericarditis have been reported (Table 1), but the most common in Spain is idiopathic or viral pericarditis, particularly among outpatients, in whom this etiology accounts for more than 90% of all cases. The terms "viral" and "idiopathic" are used almost interchangeably, as most cases of "idiopathic" pericarditis are actually of viral origin. (The actual etiology is not normally investigated in everyday clinical practice because of limitations in laboratory techniques and the limited repercussion the findings have on the management of the disease.) Pericarditis secondary to myocardial infarction, heart surgery, renal impairment, or neoplastic diseases is relatively common in the hospital setting. Tuberculous pericarditis and purulent pericarditis are very uncommon in Spain, but tuberculous pericarditis is the most common cause of pericarditis in some regions of the world (for example, sub-Saharan Africa).
Clinical Manifestations
The basic clinical symptom of acute pericarditis is chest pain. The onset of pain usually occurs relatively rapidly, but not as rapidly as in an acute myocardial infarction. Pain will be prolonged (generally lasting several days), and located in the precordial or retrosternal region, but may radiate to the neck, back, and left shoulder and arm. Pain will also often spread to the supraclavicular region and the trapezial region due to involvement of the phrenic nerves that enter the diaphragm. Pain may be exacerbated by breathing in, chest movements, decubitus position, and coughing but eased by sitting with the trunk leaning forward. It should be emphasized that, even though it is fairly characteristic, the diagnosis of pericarditis cannot be established on the type of pain alone. Frequently, patients have been diagnosed with pericarditis solely on the grounds of their pain, and often because of relatively nonspecific chest pain. Other common symptoms are dyspnea, which not only affects patients with cardiac tamponade but also patients without hemodynamic compromise because the pain itself may limit deep breathing. Fever, cough, and asthenia may also occur.
The main pathognomonic sign of acute pericarditis is pericardial friction rub, detected by auscultation in approximately 60% to 85% of the cases. Such a finding allows definitive diagnosis of acute pericarditis, but diagnosis cannot be discarded in its absence. Friction rub is a scratchy superficial sound that is heard most strongly in the mesocardium and the lower left parasternal edge and that varies in strength with respiratory movements. It is normally louder when breathing in. Typically, friction rub has 3 components (presystolic caused by atrial contraction, systolic caused by ventricular contraction, and diastolic associated with the phase of rapid ventricular filling in the protodiastole). Sometimes, just 1 or 2 components can be discerned, and the sound can therefore be confused with a murmur. Given that friction rub sounds are often evanescent, it is important to auscultate repeatedly in patients with clinical suspicion of pericarditis. Friction rub can be present in pericarditis regardless of whether effusion is present or whether effusion is extensive, even in patients with cardiac tamponade. When the pericarditis involves extensive effusion, signs of tamponade may appear. We will comment on this later in the article. Cardiac sounds may be muted when effusion is very extensive, though not always.
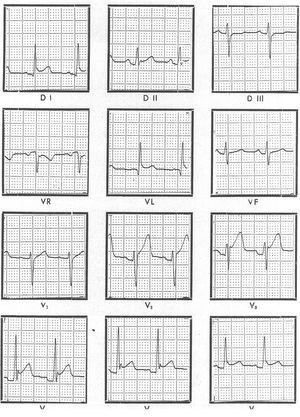
Electrocardiographic findings are abnormal in 80% of the patients with acute pericarditis.4 In the most typical cases, ECG changes can be described in 4 stages. Stage I consists of a diffuse ST-segment elevation with upward concavity (indicative of subepicardial injury) and positive T waves. The PQ or PR segments may be depressed (indicative of atrial injury) (Figure 1). These changes may last several hours or a few days. In Stage II, the ST segment returns to the isoelectric position. Stage III is characterized by the appearance of negative T waves that may return to normal in a few days, but they often remain negative for weeks or months. This should not be interpreted as persistence of the disease. Stage IV corresponds to normalization of the ECG. The changes in stage I may be confused with those of myocardial infarction and with the normal variant of repolarization known as "early repolarization." No PR-segment depression occurs in myocardial infarction, ST-segment elevation is upwardly convex and may have a mirror image in some opposing leads, and Q waves may often appear. In pericarditis, on the other hand, Q waves do not appear and arrhythmias other than sinus tachycardia are rare. Early repolarization is a normal variant characterized by ST-segment elevation with upward concavity and positive T waves that resemble changes seen in acute pericarditis. The most reliable differential finding is the ratio of the ST-segment elevation to T-wave amplitude in the V6 lead: pericarditis is indicated when the ratio is greater than 0.24. Nevertheless, the normal variant of early repolarization can be definitively distinguished from acute pericarditis in the ECG if the ST segment changes over time, because the ST segment remains unchanged in early repolarization. In the event of extensive pericardial effusion, the amplitude of the QRS complex may decrease or follow cyclic changes (electrical alternation), particularly in patients with tamponade.
Figure 1. Electrocardiogram of a patient with acute idiopathic pericarditis showing diffuse elevation of the ST segment with upwards concavity and positive T waves (stage I) and PR-segment depression at leads V2-V4, which indicates the presence of a atrial injury curve.
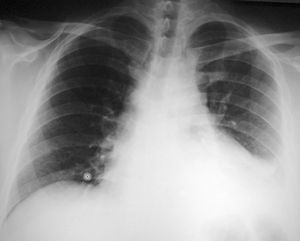
In pericarditis with no or mild effusion, radiological images of the heart appear normal. Cardiomegaly appears when effusion exceeds 250 mL. In acute pericarditis, pleural effusion, and left pleural effusion in particular, is common (Figure 2).
Figure 2. X-ray image showing the presence of cardiomegalia (caused by pericardial effusion) and left pleural effusion. The image is taken from a patient with idiopathic pleuropericarditis.
Echocardiography is the most useful diagnostic technique for identifying the presence of pericardial effusion and quantifying its extent. Nevertheless, echocardiography should not be considered as essential for establishing diagnosis of acute pericarditis, which, as mentioned earlier, should be based on other criteria. The course of acute pericarditis may not include effusion and, in contrast, not all cases of pericardial effusion are due to acute pericarditis. Echocardiography can also provide very useful information for establishing diagnosis of tamponade, essentially by detecting right atrial or ventricular collapse and abnormal mitral and tricuspid valve flow.
Specific etiologies aside (uremia, leukemia), general blood analysis will usually only provide nonspecific information (increased erythrocyte sedimentation rate). In some cases, the markers of myocardial injury may be slightly elevated due to epicardial inflammation or involvement of adjacent myocardium. Elevation of creatine kinase or its MB fraction (CK-MB) is very uncommon, but patients with pericarditis may have troponin elevations of between 35% and 50%.5
Differential Diagnosis
The signs and symptoms of pericarditis may be confused with ST-segment elevation myocardial infarction during the first few hours after onset. Nevertheless, certain features will generally allow correct differential diagnosis (Table 2). Dissecting aortic aneurysm is rarely mistaken for pericarditis when the case history is recorded correctly because, unlike pericarditis, the onset of pain is sharp, very intense, and located in the back rather than the precordial region. It should be remembered, however, that an aortic aneurysm may hemorrhage into the pericardial sac, leading to cardiac tamponade. Pleuritic pain has some elements in common with pericarditis, but the pain is located at the sides of the chest. Pericarditis may coexist with pleuritis (pleuropericarditis), and pain therefore has mixed characteristics. The intensity of pain originating in the chest wall also varies with the intensity of movement, but the sensitivity of pain to local pressure allows diagnosis to be made.
Patients are often observed with pain in the anterior plane of the chest. The characteristics of this pain are compatible with pericarditis, but no other objective findings in the physical examination or the ECG that support diagnosis of pericarditis may be present. In such cases, it is preferable not to establish diagnosis of pericarditis until the findings from further tests are available.
Etiologic Diagnosis
Once the clinical diagnosis of pericarditis has been made, the next step should be to try to establish the causes. It is first necessary to establish whether the patient has some underlying disease that could have caused pericarditis. Patients with renal impairment, recent myocardial infarction, heart surgery, chest radiotherapy, or known neoplastic disease may develop acute pericarditis which is of course classed as secondary to the underlying disease.6 Such causes are usually reported in hospital patients. In contrast, outpatients generally have primary pericarditis, and a large majority of these (more than 90%) have idiopathic or viral pericarditis. The term "idiopathic" applied to acute pericarditis describes the most common form of this disease. Sufficient evidence is available to suggest that most cases of idiopathic pericarditis are in fact due to viral infection or an immunological response to viral infection. The complex techniques, cost, and invasive tests needed to objectively determine etiologic diagnosis (determination of immunoglobulin [Ig] G, IgM, IgA, and complements in pericardial fluid, pericardial and epicardial biopsy, virological, and immunohistochemical studies) are in no way compensated by findings that may be unreliable. In any case, this type of pericarditis resolves spontaneously in most patients. Recently, the term "autoreactive pericarditis" has been introduced to describe patients with identification of elements indicative of an autoimmune response who would otherwise be classed as idiopathic.7 Our group disagrees with the use of this term for 2 reasons. First, the exact criteria for acceptance of these immunological findings as defining a specific form of pericardial disease have not been established. Second, the process for demonstrating this etiology is extremely complex and it is not clear that clinical decisions would be affected.
A series of clinical data are available that may be very useful for guiding etiologic diagnosis of acute pericarditis. Pericarditis that resolves in a few days is almost always of idiopathic/viral origin, and diagnosis becomes definitive if pericarditis recurs with clear asymptomatic periods between episodes. Tests are necessary to discard purulent pericarditis in the presence of bacterial chest infections (pneumonia, empyema, or mediastinitis) or subphrenic infection.8 In contrast, other clinical findings, such as prolonged clinical course or presence of pericardial effusion or tamponade are nonspecific. Idiopathic/viral pericarditis can follow a prolonged clinical course and may present concurrently with tamponade. In fact, although tamponade arises relatively more frequently in specific pericarditis, it is still not that rare in idiopathic/viral pericarditis, and is reported in around 15% of the patients who are admitted to the hospital. As most cases of acute pericarditis are of idiopathic/viral origin, this is the most common cause of acute pericarditis with concurrent tamponade in absolute terms. In a series of 231 patients with acute primary pericardial disease, 22 out of 24 with specific pericarditis (tuberculous, purulent, or neoplastic) had pericardial effusion and, of these, 15 (68%) had tamponade, whereas only 102 of 207 patients with pericarditis of other etiologies had pericardial effusion and, of these, 29 (28%) developed tamponade.9 Therefore, of the set of 44 patients with tamponade, the most common cause (29 of 44) was idiopathic pericarditis. A further finding that can help etiologic diagnosis is pleural effusion, which can be present both in specific pericarditis and in viral or idiopathic pericarditis.
The main concepts of any protocol for diagnostic management of acute pericarditis are: 1) knowledge of the epidemiological distribution of etiologies according to the geographic region; 2) importance of identifying some specific etiologies; and 3) knowledge of the diagnostic efficiency of invasive examinations of the pericardium (pericardiocentesis and pericardial biopsy). Tuberculous pericarditis is the clearest example of the importance of applying the first concept for diagnostic and therapeutic management of acute pericarditis. In Spain, tuberculous pericarditis occurs very infrequently (accounting for around 4% of the cases of pericarditis),9,10 but in some regions (such as sub-Saharan Africa), tuberculosis is the most common cause of pericarditis (accounting for around 70%-80% of cases and for 90%-100% in patients who also have AIDS).11 Thus, in Spain, acute pericarditis is not considered tuberculous unless shown otherwise, but in other countries and in some patient groups, administration of antituberculosis treatment right from the start could be justified. The second concept is also important. It is not particularly relevant to establish diagnosis of viral pericarditis (through isolation of the virus, serological analysis, or polymerase chain reaction [PCR] techniques) because the therapeutic management and disease course are similar to those of idiopathic pericarditis and, in the case of neoplastic pericarditis, effective treatment is not usually possible (except for managing tamponade). In contrast, identification of tuberculous and purulent pericarditis is obviously important. The findings from pericardiocentesis and pericardial biopsy can definitively identify purulent, tuberculous, and neoplastic pericarditis. Overall, the diagnostic efficiency of pericardiocentesis and pericardial biopsy is 19% and 22%, respectively, but there is a significant difference between these 2 procedures when they are performed for purely diagnostic reasons (that is, in patients without hemodynamic compromise) (efficiency of 6%) or when done for therapeutic reasons in patients with tamponade (efficiency of 35%). This is probably because proportionately more patients with specific causes of pericarditis are included in this group.9
In accordance with these considerations, we propose the following protocol for diagnosis and management of pericardial diseases.12,13 This protocol, whose good diagnostic efficiency has been validated in successive studies,6,14 has been used by us with slight changes for more than 2 decades. It is based on the strong prevalence of viral or idiopathic pericarditis, for which specific treatment is unavailable, and aims to compromise between limiting the number of unnecessary invasive examinations and performing as many specific diagnostic tests as possible. We divide this protocol into 3 stages which are presented below.
Stage I: General Studies and Echocardiography
A complete clinical history is taken and a physical examination, ECG, chest x-ray, echocardiogram, and general blood analysis are done in the first stage of our protocol. Antinuclear antibodies are measured in patients with tamponade or pericarditis with pericardial effusion of more than 1 week duration. The presence of Mycobacterium tuberculosis is also tested in these patients in 3 sputum or gastric aspirate samples. We do not carry out virological studies because of their limited practical relevance. Although cases of pericarditis have been reported due to a wide range of infectious agents (mononucleosis, Mycoplasma pneumoniae, Legionella, Coxiella, etc), such causes are uncommon. Therefore, systematic and indiscriminate investigation in all patients with acute pericarditis is only justified in specific epidemiological situations or when other suggestive findings are present (lymphadenopathy, pulmonary infiltrates, etc). At this stage of the protocol, examinations aim to produce specific findings (for example, lymph biopsy in presence of lymphadenopathy, bronchoscopy in patients with pulmonary masses). Whenever patients present with pleural effusion, it is important to sample fluids to measure adenosine deaminase (ADA)--ADA levels above 45 U suggest tuberculosis but low levels allow this disease to be completely discarded. This simple analysis is therefore very useful as a screening method for deciding whether to proceed with other examinations. Nevertheless, when venous hypertension is present, pleural effusion may be due to transudate, in which case false low values of ADA would be obtained. In certain instances, PCR assays for M tuberculosis may be useful.
Stage II: Pericardiocentesis
Pericardiocentesis should only be done when the patient presents with cardiac tamponade (that is, for therapeutic reasons), or when purulent pericarditis is suspected. The hematocrit level (in the case of hemorrhagic effusion), protein concentration, and ADA levels in pericardial fluid should be determined,15 cytology should be done, and samples should be cultured for aerobic and anaerobic bacteria and M tuberculosis, preferably with a fast cell culture system (BACTEC). In addition, whenever tuberculous pericarditis is suspected, a fluid sample should be stored for PCR assays, as the positive and negative predictive value of such procedures is high,16 even though relatively little information is available on the diagnostic precision of this test. Likewise, whenever neoplastic pericarditis is suspected, determination of the carcinoembryonic antigen (CEA) in pericardial fluid may be useful.15 In Spain, pericardiocentesis is not indicated when patients present with pericardial effusion unless signs of tamponade are also present, because the diagnostic efficiency is poor in such cases.9
Stage III: Pericardial Biopsy
Pericardial biopsy should only be done in patients with recurrent tamponade after pericardiocentesis (a procedure which also involves pericardial drainage) and in patients with pericardial effusion and persistent clinical symptoms without an etiologic diagnosis 3 weeks after hospital admission. Pericardial biopsy could also be indicated in patients with a high suspicion of tuberculous pericarditis but who lack of a definitive diagnosis (for example, frankly elevated ADA in the pleural or pericardial fluid but no other signs).
The rationale behind this approach to restricting the indications for pericardial procedures with purely diagnostic ends is that most cases of acute pericarditis in Spain are idiopathic, even in presence of effusion, tamponade, and a prolonged clinical course.
The recent "Guidelines for Clinical Practice," drawn up by the European Society of Cardiology, and later published as an executive summary by Revista Española de Cardiología,17 also propose a systematic approach to the diagnosis of the cause of pericarditis. However, attentive analysis of these guidelines shows that our approach has two substantial and fundamental differences with regard to use of invasive examinations. First, the liberal use of pericardial fluid studies and pericardial and epicardial biopsy is not present in our protocol, and second, we do not recommend widespread use of pericardioscopy. Some authors favor use of pericardioscopy for pericardial biopsy in accordance with the rationale that the diagnostic efficiency is high for viral pericarditis, autoreactive pericarditis, and neoplastic pericarditis.18 Pericardioscopy may indeed have a slightly higher sensitivity and specificity than pericardiocentesis and conventional pericardial biopsy in certain circumstances, but this does not justify extending the general indications of pericardial drainage given that the aforementioned predominance of idiopathic pericarditis renders many of these examinations unnecessary. We have already mentioned that demonstrating a viral origin or elucidating the immunological mechanisms of acute pericarditis has few practical implications. On the other hand, diagnosis of neoplastic pericarditis can be established in many patients with modern imaging techniques, cytological study of pericardial fluid, and conventional pericardial biopsy. Pericardioscopy, in contrast, is a technique of limited availability with a steep learning curve and a certain degree of risk if a rigid device is used. Thus, in our opinion, the "Guidelines for Clinical Practice" of the European Society of Cardiology risk unnecessarily extending the use of invasive examinations in many patients with pericarditis with a benign and self-limiting course.
Pericarditis that presents in patients with AIDS is worthy of special mention.19 These patients frequently present with pericardial effusion, usually in an advanced stage, and so the overall prognosis is poor. Nevertheless, cardiac tamponade is uncommon. Pericardial involvement can be caused by infectious agents such as the acquired human immunodeficiency virus (HIV) itself, other viruses (herpes simplex, cytomegalovirus), bacteria, and fungi, or by neoplastic processes (lymphoma, Kaposi sarcoma). Most of the cases of pericarditis in Spain are, however, nonspecific and so management of these patients should be similar to the general management discussed earlier.
Treatment
Patients with acute idiopathic or viral pericarditis should rest in bed or in an arm chair while inflammatory symptoms persist (pericardial pain and fever). Most patients can probably be treated in an outpatient setting, provided they can be closely monitored by medical personnel who are familiar with the disease. Hospital admission should be reserved for patients with high fever, subacute clinical course, cardiac tamponade, severe effusion, myocardial involvement, and for immunodepressed patients or those who are receiving anticoagulant treatment.20 Pharmacological treatment consists of administration of aspirin or other nonsteroidal antiinflammatory drugs. The first-choice drug is aspirin, and the initial dose is 500 or 1000 mg every 6 hours. This dose should be maintained while pain and fever last. Once symptoms have remitted, the dose can be gradually reduced (for example, 500 mg every 8 hours for a week and then 250 mg every 8-12 hours for a further 2 weeks). If the patient does not respond to aspirin, or if aspirin is contraindicated, nonsteroidal antiinflammatory agents can be used (for example, indomethacin 75-225 mg/day, paracetamol 2-4 g/day, or ibuprofen 1600-3200 mg/day, alone, or in combination with each other or with aspirin). Diclofenac can be used as an additional measure to calm breakthrough pain. It may also be useful to apply a bag of ice to the chest. Corticosteroids are not first-choice drugs; in fact, they should be avoided as most as possible. It is preferable to provide psychological support for the patients and encourage them to put up with the pain for a few more days rather than resort prematurely to administration of corticosteroids. Whereas corticosteroids can rapidly control symptoms in most patients, they are also associated with the appearance of relapses. In fact, some patients present with repeated relapses every time they try to reduce the dose, and so become "hooked" on corticosteroids with resulting exposure to the side effects of these drugs. Corticosteroids should therefore only be considered in patients with persistent severe pain and high fever that has lasted for more than 7 or 10 days and that is refractory to the other drugs mentioned earlier, and provided tuberculosis has been discarded. According to our experience, the use of corticosteroids is rarely necessary if the anti-inflammatory and analgesic agents mentioned above are appropriately used. Should they be administered, however, the period of treatment should be at least 2 to 4 weeks. The initial dose (40-60 mg of prednisone or equivalent) should be maintained while pain, fever, or extensive effusion persist, and then tapered off gradually in order to eliminate them entirely within 4 to 6 weeks.
The specific forms of pericarditis should be treated in accordance with the cause. Aspirin is the first-choice drug in patients who have recently suffered a myocardial infarction, whereas indomethacin should be avoided in patients with acute ischemic heart disease, as it reduces coronary artery flow. Treatment of purulent pericarditis requires surgical drainage of the pericardium, in addition to the administration of appropriate antibiotics. Tuberculous pericarditis should be treated with the same drugs as those used for pulmonary tuberculosis. The efficacy of corticosteroids for preventing possible progression towards pericardial constriction has not been clearly proven. We emphasize that antituberculous treatment should be administered only when diagnosis is completely certain (M tuberculosis has been isolated from the pericardial fluid or other sites, or in the presence of caseated granulomas in the pericardial or other tissues). Under no circumstances should antituberculous treatment be administered without a good reason.
RECURRENT PERICARDITIS
Between 8% and 80% and, on average, around 24% of patients with acute pericarditis will suffer recurrences. Usually, most of these patients will have a single recurrence, within the first weeks after the first episode, but some may suffer from repeated episodes for months or years.12,21 Sometimes, symptoms reappear every time antiinflammatory treatment is withdrawn or within 6 weeks of the initial exacerbation. These cases are described as "incessant pericarditis." Although these relapses can occur after withdrawal of the usual antiinflammatory drugs, it is particularly common and bothersome in patients who have received corticosteroids. Some patients have a threshold dose of prednisone below which a relapse is probable (generally between 10 and 20 mg). The term "intermittent pericarditis" refers to patients with symptom-free intervals of more than 6 weeks without treatment.
The most typical form of recurrent pericarditis is a second episode after a first episode of idiopathic pericarditis, presumably of viral origin. The pathogenesis of this syndrome may be associated with persistent or recurrent viral infection, with an immunopathological mechanism, or with an inappropriate pharmacological treatment. It has been suggested that treatment with corticosteroids during the initial exacerbation may increase the likelihood of recurrence because of the negative effect of the drug on viral replication. In particular, an immunological mechanism is most likely in patients with systemic lupus erythematosus having recurrent pericarditis. Pericarditis may also recur after myocardial infarction or heart surgery--relapses after heart surgery are more common in children and adolescents, particularly after closure of an atrial shunt.22 Infectious pericarditis (purulent and tuberculous) can follow an acute, subacute, or chronic course with persistent symptoms, but it does not present with the symptoms of true recurrent pericarditis. Likewise, neoplastic pericarditis follows a persistent course and, in general, progression of the underlying neoplastic disease does not allow a chronic course. Nevertheless, on exceptional occasions, some cases of neoplastic pericarditis present initially with an apparently self-limiting episode of acute pericarditis, with subsequent recurrence of pericardial manifestations.23 Thus, relapsing-remitting pericarditis with asymptomatic periods of longer than 6 weeks can be attributed to idiopathic pericarditis in all certainty without the need for additional examinations, provided patients have not undergone heart surgery and provided lupus erythematosus has been discarded.
Although the manifestations during relapses are similar to those of the first episode of pericarditis, the first episode is characteristically the most severe, and subsequent episodes tend to be clinically less severe. In particular, objective signs of pericarditis (pericardial friction rub, electrocardiographic changes, and pericardial effusion) are much more common in the initial episode and are often absent in subsequent episodes, which present with "pericardial pain" only. It can therefore be difficult to establish whether the patient is really presenting with a new episode. If pericardial effusion is not present during the first episode, it will probably be absent from subsequent episodes. Likewise, tamponade is very uncommon in relapses. The number of recurrences and the interval between episodes vary greatly from patient to patient and are hard to predict. In our experience with 44 patients with recurrent pericarditis who had not received corticosteroids, 20 had 2 episodes, 19 had 3 to 5 episodes, and 5 had more than 5 episodes.2 The time between episodes varies greatly. In the series of Fowler and Harbin,24 half the patients (15 out of 31) had asymptomatic periods of 1 year or longer and 12 patients were asymptomatic for 2 years or more (2 patients were even asymptomatic for 8 years). Nevertheless, in general, episodes become less frequent and progressively less severe. As previously mentioned, tamponade is very uncommon and progression to constrictive pericarditis is extremely rare.
When managing these patients, it is first necessary to assess whether treatment of the first episode was appropriate or inappropriate (rest time too short, low doses of antiinflammatory agents, premature interruption of these agents). In general, treatment of recurrences should be the same as for the first episode of pericarditis. It is important for the patient to rest until fever and chest pain have disappeared, and to administer nonsteroidal antiinflammatory drugs at the appropriate doses following the treatment regimens described in the previous section. Once again, we emphasize that use of corticosteroids should be avoided.
In patients who have presented with 2 or more recurrences or in patients with incessant pericarditis, initial treatment with colchicine is indicated (in association with nonsteroidal antiinflammatory drugs). Ever since the initial study of Rodríguez de la Serna et al25 in 1987, clinical experience has been mounting to support the efficacy of colchicine in treatment of episodes of recurrent pericarditis. In the most important multicenter study,26 51 patients were included (33 with idiopathic pericarditis and 18 with secondary pericarditis) and treated with colchicine for 6 to 128 months (mean, 36 months). It is of note that 29 of the patients received corticosteroids. Before treatment with colchicine, the number of recurrences ranged from 2 to 15 (mean, 3.5 per patient) with an interval between episodes of 2 months. For a total period of 1004 patient-months (mean, 12 months per patient) of treatment with colchicine (starting dose of 0.5-3 mg/day and maintenance dose of 0.5-2 mg/day), only 7 (13.7%) of the 51 patients presented recurrences. Colchicine was withdrawn in 39 patients and 14 of them presented with relapses which, in general, were not very severe and could be controlled in all patients by reinitiating treatment with colchicine. During the 2333 patient-months of follow up, 31 patients (60.7%) remained free of relapses. Although these observations are important, this was not a prospective, randomized, double-blind study. Moreover, the large variability in the intervals between episodes in these patients and doubts about patient selection question the validity of these findings. It should also be noted that other investigators have not found colchicine to be effective in their patients.23
The true efficacy of colchicine is not known, as no prospective, placebo-controlled studies have been done. Our impression is that colchicine is actually useful in more than half the patients and we advise its administration in patients with 2 or more recurrences. The recommended starting dose is 1 mg every 12 hours, a dose which can be reduced to 0.5 mg every 12 hours in patients with digestive intolerance. The recommended duration of treatment with colchicine (0.5-1 mg/day, according to the patient's weight and tolerance of the drug) is 1 year.
If the patient is receiving treatment with corticosteroids for any reason and presents with relapses when corticosteroid treatment is interrupted, maximum effort should be made to control episodes with aspirin or nonsteroidal antiinflammatory agents (alone or in combination) and further increases in corticosteroid dose should be avoided if possible. As mentioned earlier, colchicine (1-2 mg/day) can be useful during withdrawal from corticosteroids. In these patients, reduction of corticosteroid dose should be done slowly (1.25-2.5 mg/month).
Contrary to the recommendation of avoiding corticosteroids in patients with pericarditis, some authors have supported the opposite approach, which is, administration of high-dose corticosteroids.27 The pathophysiological rationale for this recommendation is that the immunodepressant effect of high-dose corticosteroids inhibits T-cell mediated cytolysis, which may play a role in recurrent episodes of pericarditis. Likewise, immunodepressor treatment (azathioprine 75-100 mg/day) or pericardiectomy can be considered in refractory patients with a long course (>1 year) recurrence of pericarditis who have presented with multiple episodes (>6) that seriously limit their quality of life. However, immunodepressant treatment is relatively untried, and pericardiectomy is often ineffective. These therapeutic options should therefore only be considered in exceptional cases and the clinician should be sure that all other therapeutic alternatives have been tried. We ourselves have never administered immunodepressants and we have only done pericardiectomy in very few patients with variable results. In fact, patients may continue to have episodes of pericarditis, because complete pericardiectomy is impossible. Our impression, in line with the experience of other authors,24 is that in patients with benign recurrent acute pericarditis, the appropriate use of the above mentioned drugs can satisfactorily control pericarditis in most cases, and that the disease eventually resolves.
The role of physical activity in recurrences and relapses of pericarditis is unknown. In clinical practice, it is relatively common to find patients who report worsening of symptoms with exercise, particularly if the only manifestation of the disease is persistent precordial pain. It is much less clear whether exercise can act as the trigger of well-defined episodes of pericarditis (with pain, fever, and friction rub). In any case, it seems reasonable to limit physical exercise, especially when corticosteroid or antiinflammatory treatment is being withdrawn. The extent to which exercise should be limited is hard to establish, but it is recommended to restrict physical exercise to a level where the patient can carry out domestic activities and sedentary work.
CARDIAC TAMPONADE
Cardiac tamponade is caused by compression of the heart due to pericardial effusion. Tamponade is not an "all or nothing" condition as was thought for some years, but rather its severity ranges from a slight increase in intrapericardial pressure with minimal repercussion on cardiac function (and no clinical manifestation) to severe hemodynamic compromise that may even be fatal.28
Tamponade can present in any type of pericarditis but it is proportionately more common in neoplastic, tuberculous, and purulent pericarditis than in viral or idiopathic pericarditis. However, as mentioned earlier, in absolute terms, the most common cause of tamponade in patients who have received medical treatment only (patients who have not undergone any surgery or invasive procedures and who have no chest trauma) is acute idiopathic pericarditis because of its higher prevalence. These patients normally have clear inflammatory signs and symptoms (fever, chest pain, friction rub). In contrast, these signs and symptoms are absent in many patients with tamponade of neoplastic origin, therefore idiopathic pericarditis can be discarded in cases of isolated tamponade.6 Type A aortic dissection that tears the pericardial sac is another cause of tamponade, but its overall clinical presentation is sufficiently characteristic for such a cause to be suspected.
Patients with tamponade typically present with dyspnea and chest pain. They can also present with syncope, particularly when tamponade is acute, such as when caused by aortic dissection. The clinical signs of tamponade consist of jugular engorgement, hepatomegalia, pulsus paradoxus (decrease in systolic blood pressure by more than 10 mm Hg on inspiration during spontaneous respiration) and, in severe cases, arterial hypotension and shock. None of these signs is pathognomonic of tamponade, but if they are present (in particular, pulsus paradoxus) tests must be done to discard it, particularly when there are no concomitant signs of left heart failure (which is very rarely present in tamponade). If uncertain, Doppler echocardiography can be of great help because the presence of pericardial effusion with signs of hemodynamic compromise (collapse of right chambers, exaggerated changes in mitral and tricuspid flows during respiration) has a very high predictive value for diagnosis of tamponade. If, on the other hand, such signs are absent, diagnosis should be reconsidered. Iatrogenic cardiac tamponade is a particular situation that may present after heart surgery. Diagnosis is difficult and may require other imaging techniques for diagnosis, such as computed tomography. Tamponade with clinical symptoms is the most severe manifestation. Patients with moderate or severe pericardial effusion may present relatively frequently with some echocardiographic signs of tamponade (in particular, right atrial and/or ventricular collapse) but without any clinical signs of hemodynamic compromise. Whereas absence of echocardiographic signs has a very high negative predictive value for tamponade, the positive predictive value of these findings for diagnosis of clinical tamponade (in particular, isolated collapse of the right atrium) is very low, around 30%.29 Mild hemodynamic compromise may result from tamponade, but this in itself does not require pericardial drainage.
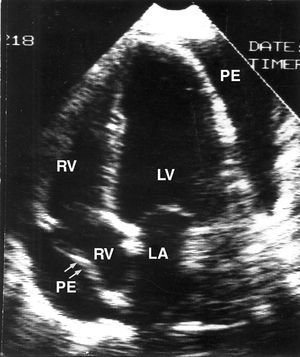
If patients present with severe cardiac tamponade (hypotension, low cardiac output, or shock), pericardial drainage should be done. Whether pericardiocentesis or surgical drainage is preferred depends on the preferences and experience of the treating physician and on the structure of the hospital itself, for example, whether an attending cardiologist or a surgical team is available. Our approach is to start with subxiphoid pericardiocentesis, and only resort to surgical drainage for cases in which pericardiocentesis has been ineffective (for technical reasons or because of the characteristics of the fluid itself) or in cases of recurrence. In patients with mild or moderate tamponade who are unlikely to have specific forms of pericarditis, a compromised approach can initially be adopted.28 Many patients with acute idiopathic pericarditis and whose tamponade is not very severe progress well with rest and antiinflammatory agents, and pericardiocentesis is often not necessary (Figure 3). Patients with neoplastic pericardial effusion require a different approach, as effusion in this case will probably worsen and aggravate the tamponade. Pericardial drainage is therefore justified even in patients whose tamponade is not particularly severe. The most appropriate type of pericardial drainage is subject to debate. In principle, less aggressive procedures are preferred (above all in patients with poor overall prognosis) but at the same time, they must be able to prevent reappearance of effusion. Simple needle pericardiocentesis can often resolve the tamponade initially, but the probability of relapse is very high. On the other hand, surgical drainage (or pericardiectomy, its major equivalent) is excessive for many patients. The best option is pericardiocentesis with the Seldinger technique, leaving a pigtail drainage catheter that should be kept in place until drainage is complete.30 If effusion recurs after withdrawal of the pigtail catheter, a sclerosing agent (tetracycline or bleomycin) can be instilled into the pericardial sac, or a subxiphoid balloon pericardiotomy can be done.30
Figure 3. Two-dimensional echocardiogram of a patient with acute pericarditis and substantial pericardial effusion with right-atrial collapse. The patient had acute idiopathic pericarditis and presented with not very severe clinical signs of tamponade. The patient progressed well with nonsteroidal antiinflammatory treatment and pericardiocentesis was not required.
Treatment of pericardial effusion also requires special consideration in patients submitted to hemodialysis. Even if severe tamponade is not present, these patients may poorly tolerate hypovolemia that arises during hemodialysis sessions, and so it is often necessary to resort to pericardial drainage.
(ACUTE AND SUBACUTE) CONSTRICTIVE PERICARDITIS
This article is not intended to describe constrictive pericarditis into details, although this section will comment on some forms of constriction related to acute pericarditis.
While exudative acute idiopathic pericarditis is resolving, signs of pericardial constriction are relatively common--reported in up to 30% of the patients in some studies.31,32 These signs are generally subclinical (high jugular venous pressure with rapid "Y" descent, pericardial knock, ventricular septal notch in the echocardiogram, and abnormal venous flows), but some patients may present with signs of right heart failure. In most patients, these signs resolve without special measures (and without corticosteroids) in a few weeks ("transient cardiac constriction"). Idiopathic or viral pericarditis therefore rarely progresses to severe and persistent constrictive pericarditis requiring pericardiectomy (only about 1% of the patients undergo such a procedure), whereas 50% of the patients with tuberculous pericarditis and 30% of those with purulent pericarditis require pericardiectomy because of progression to constrictive pericarditis. These forms of constrictive pericarditis present fairly early, usually after a few weeks or in the first 3 months after the phase of exudative pericarditis and may be very acute, particularly in the case of purulent pericarditis. In some patients, constriction even appears during the exudative phase and tamponade may coexist with constriction--the exudative-constrictive form of pericarditis.33 Substantial participation from the visceral pericardium is present in these forms of constriction ("constrictive epicarditis"), and should be recognized if pericardiectomy is to be performed.
Correspondence: Dr. J. Sagristà Sauleda.
Servei de Cardiologia. Hospital General Universitari de la Vall d'Hebron.
Passeig de la Vall d'Hebron, 118-128. 08035 Barcelona. España.
E-mail: jsagrist@vhebron.net