Keywords
INTRODUCTION
Basic and clinical research carried out in recent years has established a direct, 2-way relationship between chronic kidney disease and cardiovascular disease.1,2 In daily clinical practice, serum creatinine concentrations and the glomerular filtration rate estimated with the Cockcroft-Gault3 formula or Modification of Diet in Renal Disease (MDRD)4 equation are commonly used to estimate renal function. However, plasma creatinine concentrations can be influenced by a number of factors, such as patient age, sex, muscle mass, physical activity, diet, and medication.5
Cystatin C is a protein inhibitor of cysteine protease that is synthesized at a stable rate by all nucleated cells. Because of its low molecular weight and high isoelectric point, it can be eliminated almost exclusively by glomerular filtration. Cystatin C concentrations are not influenced by age, sex, or protein ingestion, and they are sensitive to small changes in glomerular filtration. Because of these characteristics, plasma cystatin C concentration is considered among the best markers of glomerular filtration status.6-9 Recently, several studies have reported an association between elevated cystatin C values and the development of cardiovascular complications in patients with coronary disease. It is currently not known whether this relationship is due to the fact that cystatin C is a better marker of renal function than serum creatinine or whether there are factors apart from glomerular filtration that affect the concentration of this protein and are additionally related to cardiovascular risk.10
The aim of this study was to assess the prognostic value of plasma cystatin C concentration in patients hospitalized for high-risk acute coronary syndrome (ACS), and to investigate the relationship between cystatin C and other markers of renal function and inflammation.
METHODS
This is a prospective, observational study performed between May 2006 and July 2007. A total of 203 patients older than 18 and consecutively hospitalized in the cardiac intensive care unit (ICU) of our center with a diagnosis of high-risk ACS were included.
The diagnostic criteria for high-risk ACS included at least 2 of the following factors: pain with ischemic characteristics, electrocardiographic alterations consistent with ischemia, and/ or elevated myocardial necrosis markers. The treatment provided and additional tests performed during the patient's hospital stay were based on the clinical decisions of the attending physician. In most patients, an invasive strategy was used, involving urgent coronary angiography (for myocardial infarction with ST segment elevation or left bundle branch block) or early scheduled coronary angiography during the first 24 to 72 hours following hospital admission.
Clinical Characteristics
The following data were recorded from the patients' clinical records: demographic information, presence of classic cardiovascular risk factors (diabetes mellitus, systemic hypertension, dyslipidemia, and smoking), and history of known vascular disease (ischemic heart disease, cerebrovascular disease, peripheral arterial disease, and prior myocardial revascularization treatment). The other clinical variables analyzed included the type of ACS, Killip class at the time of hospitalization, systolic and diastolic pressure values, pulse pressure (estimated as the difference between systolic and diastolic arterial pressure), and heart rate.
All patients underwent echocardiography, in which the left ventricular ejection fraction was estimated with the Simpson method using a 4-chamber approach; the mean of 3 determinations was calculated in patients with sinus rhythm, and the mean of 5 determinations in those with atrial fibrillation.
In 95% of patients, coronary angiography was performed, and the severity of coronary lesions was evaluated with a score based on the number of affected epicardial coronary arteries.
Analytical Data
Baseline hemoglobin, hematocrit, leukocytes, glucose, and creatinine values were recorded in the first laboratory analyses performed at our center at the time of hospitalization. Concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), glucose, high-sensitivity C-reactive protein, and cystatin C were recorded in the first 24 hours, together with the peak troponin I (TpI) level.
Cystatin C was measured with an automated homogeneous immunoassay using a Dade-Behring BN ProSpec nephelometer. The reagent for the analysis consisted of polystyrene particles coated with antibodies against the protein that agglutinate when mixed with samples containing cystatin C and disperse light at an intensity proportional to the concentration of the analyte.
The reference interval for serum cystatin C concentration in adults with the nephelometric method used is 0.51 to 0.95 mg/L. Concentrations of high-sensitivity C-reactive protein were also determined by immunoassay. The glomerular filtration rate was estimated with the MDRD equation.
Episodes Analyzed
The cardiovascular complications analyzed included in-hospital mortality, all-cause mortality during follow-up, development of a new myocardial infarction, defined as chest pain or equivalent anginal pain with ECG changes, or elevated tumor necrosis markers during the first 24 hours following hospitalization (patients with elevated markers after coronary interventional procedures were excluded), and development of heart failure during hospitalization or follow-up.
Clinical Follow-up
Mean follow-up was 186 (SD, 110; median, 156) days, during which time all the patients' clinical events were recorded. Follow-up was performed by telephone contact, in outpatient clinics, and by review of the patients' hospital medical records.
Statistical Analysis
All information was prospectively recorded in a database created with Microsoft Office Access 2003 SP2. The statistical analyses were performed with SPSS (Statistical Package for the Social Sciences), version 12.0. The categorical or dichotomous variables are expressed as absolute values and percentages, and were compared with the Pearson χ2 test. The continuous variables with a normal distribution are described as the mean (SD), and the Student t test was used for the comparisons between groups. Variables that did not present a Gaussian distribution were compared with the Mann-Whitney U test.
Spearman's correlation coefficient was used to evaluate the correlations of cystatin C concentration with high-sensitivity C-reactive protein and glomerular filtration rate. A logistic regression analysis was used to assess the independent role of clinical and laboratory factors with respect to cystatin C for predicting the development of cardiovascular complications during hospitalization, including the significant variables in the univariate analysis. The adjusted odds ratios and 95% confidence intervals (CI) are presented. Kaplan-Meier survival curves during follow-up were constructed and compared using the long-rank test.
To carry out a descriptive analysis of the population studied, some quantitative variables were categorized into intervals: the glomerular filtration rate (>90, 90-60, <60 mL/min), the number of affected vessels on coronary angiography into 3 intervals, and the ejection fraction (>55% and <55%). Cystatin C was categorized into 2 groups (>0.95 and <0.95 mg/L) for the univariate analysis.
A P value less than .05 was considered statistically significant.
RESULTS
From May 2006 to July 2007, 203 patients hospitalized with a diagnosis of high-risk ACS were included in the study. The mean age was 66.6 (13.16) years. Among the total, 62.1% of patients (n=126) presented non-ST-segment elevation ACS and 37.9% (n=77), ST-segment elevation ACS. The baseline characteristics of the study population are described Table 1.
The median cystatin C concentration was 1.01 (range, 0.83-1.35) mg/L, plasma creatinine at the first analysis performed was 1 (0.9-1.3) mg/L, glomerular filtration rate was 72.4 (49.12-93.73) mL/min/1.73 m2, and high-sensitivity C-reactive protein was 1.37 (0.46-5.02) mg/L. Glomerular filtration at the time of hospitalization was <60 mL/min/1.73 m2 in 32.5% of patients and serum cystatin C was >0.95 mg/L in 113 (55.7%) patients.
Patients with higher cystatin C values presented a poorer clinical profile, were older, had a high prevalence of hypertension, worse Killip class at the time of hospitalization, more severe coronary disease on coronary angiography, and higher plasma creatinine and C-reactive protein values (Table 2).
There were no significant differences between the 2 cystatin C groups regarding the coronary intervention performed; the final angiographic result yielded a similar success rate and no significant complications (3.27% for patients with cystatin C ≤0.95 and 4.09% in the other group). Analysis of the pharmacological treatment showed a substantially higher use of beta-blockers and aspirin in the group with cystatin C ≤0.95 (Table 2).
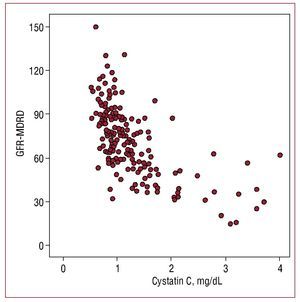
Serum cystatin C values showed a higher correlation with estimated glomerular filtration rate (r=-0.655; P=.001) (Figure 1) than with microalbuminuria (r=0.302, P=.01) and a lower correlation with C-reactive protein (r=0.29; P=.01).
Figure 1. Correlation between cystatin C values and glomerular filtration rate estimated with the MDRD formula (GFR-MDRD).
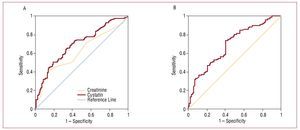
In the group of patients with cystatin C values >0.95, 6.5% presented normal glomerular filtration rates and 46%, glomerular filtration rates between 60 and 90 mL/min/1.73 m2. In addition, 10% of the patients who had renal dysfunction with a filtration rate of <60 mL/min/1.73 m2 had cystatin C values <0.95. In our sample, the cut-off point selected by our laboratory as the upper normal limit for cystatin C (0.95 mg/L) predicted cardiovascular complications during follow-up with a sensitivity of 89% and a specificity of 80.26% (Figure 2). Receiver operating characteristic (ROC) curves were performed to determine the diagnostic performance of creatinine, glomerular filtration rate estimated by MDRD, and cystatin C to identify the subgroup of patients who would present cardiovascular complications. Cystatin C showed a slightly larger area under the curve than the glomerular filtration rate; serum creatinine was the parameter with the smallest area under the curve (Figure 2, Table 3).
Figure 2. A, ROCs of cystatin C and creatinine in relation to the development of cardiovascular complications (heart failure, infarction, and cardiovascular death). B, ROC curve of the glomerular filtration rate estimated with the MDRD in relation to the development of cardiovascular complications (heart failure, infarction, and cardiovascular death).
Hospital stay lasted a median of 9 (6-19) days. From the time of admission to the completion of the study follow-up, 56 (27.58%) patients from the overall sample had developed heart failure, most of them in the group with elevated cystatin C values (45 [38.5%] patients). In-hospital mortality was significantly higher in the group with elevated cystatin C values (17.6% vs 3.3%; P=.001), and these differences persisted during follow-up (Figure 3, Table 4).
Figure 3. Association between cystatin C <0.95 mg/L and >0.95 mg/L and the development of in-hospital cardiovascular events.
In the univariate analysis, no association was observed between cardiovascular complications and the type of ACS, hyperlipidemia, or peak TpI concentration, but an association was found with the remaining cardiovascular risk factors: number of affected vessels, ejection fraction, renal function markers, cystatin C, and high-sensitivity C-reactive protein. When results from patients with a glomerular filtration rate of >60 mL/min/1.73 m2 were analyzed, we found that patients with cystatin C >0.95 mg/L presented a significantly higher rate of cardiovascular complications than patients with glomerular filtration >60 and cystatin C <0.95 mg/L (Table 5). Despite the fact that the former patients had slightly lower or normal glomerular filtration, they presented a significantly higher percentage of cardiovascular events (heart failure, infarction, and deaths due to a cardiovascular cause) than those with a glomerular filtration rate >60 and cystatin C <0.95 mg/L.
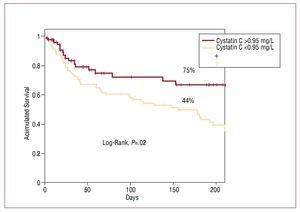
Estimated event-free survival (ie, no heart failure, myocardial infarction, or death) following a mean follow-up of 186 days was significantly higher in the group of patients with cystatin values ≤0.95 mg/L: 75% versus 44% (P=.02) (Figure 4).
Figure 4. Kaplan-Meier curves to estimate the probability of survival free from major cardiovascular events, according to cystatin C value.
The multivariate analysis identified age, ejection fraction, and cystatin C concentration as independent predictive factors of cardiovascular complications during hospitalization (Table 6).
DISCUSSION
The results of the present study indicate that elevated cystatin C values predict the development of in-hospital heart failure, myocardial infarction, and cardiovascular death in patients with high-risk ACS, independently of other classic risk factors. In addition, plasma cystatin C value may have a greater capacity to stratify patients at a high risk of cardiovascular complications during hospitalization than other methods of assessing renal function. We found that elevated cystatin C was associated with a poorer cardiovascular prognosis even in the group of patients with normal glomerular filtration. To our knowledge, this is the first report of this association, which we believe could have implications for risk stratification in this patient population.
In recent years, several articles have described a close association between renal dysfunction and cardiovascular complications during follow-up of patients with acute and chronic ischemic heart disease, as well as other clinical presentations of cardiovascular disease, in particular, heart failure.1,10-12 A reduction in glomerular filtration below 60 mL/min is related to a significant increase in the risk of death, myocardial infarction, and stroke, both in patients with or without prior cardiovascular disease.13 Specifically, this relationship has been described in ACS patients with ST-segment elevation and those without.10,13-15 In the VALIANT study16 (patients with ventricular dysfunction and heart failure following ST-segment-elevation myocardial infarction), deterioration of renal function (assessed with the glomerular filtration rate) was associated with an increase in mortality and cardiovascular complications during follow-up. Similar results have been reported for the patient populations included in the SAVE (postinfarction ventricular dysfunction), HOPE (patients with a high cardiovascular risk, the majority with chronic ischemic heart disease), and PEACE (patients with chronic ischemic heart disease) studies. In these studies, the relationship between renal dysfunction and the prognosis was observed for decreased glomerular filtration rates as well as for urinary albumin excretion.16-19
As mentioned above, a direct 2-way relationship has been described between renal dysfunction and cardiovascular disease. Chronic renal failure favors the development of hypertension and dyslipidemia, and promotes activation of the renin-angiotensinaldosterone system. These factors, together with the increase in inflammatory mediators, seem to contribute to higher production of free radicals, which intervene in the atherosclerotic process and cardiovascular injury. In addition, mineral metabolism alterations (increase of the promotors and decrease of the inhibitors of calcification) favor calcium deposits in the coronary vessels.20,21 Lastly, less intensive use of therapeutic interventions of recognized clinical and prognostic benefit has been described in patients with renal dysfunction. Specifically, in those with ACS, less aggressive treatment and longer delays in performing interventional coronary procedures, as well as lower use of IIb/IIIa, blockers of the reninangiotensin-aldosterone axis, beta blockers, statins, and antiplatelet agents, may contribute to the poorer prognosis described in this patient population.22
In daily clinical practice, assessment of renal function is usually based on serum creatinine determinations and estimates of the glomerular filtration rate using the Cockcroft-Gault or MDRD formulas. Both these methods present several limitations, many of which are derived from factors that affect creatinine production, such as age, female sex, race-related characteristics, diet, and the course of chronic diseases.
These equations were recently evaluated in patients with heart failure and compared with NT-proBNP, a prognostic marker. The prognostic information provided by NT-proBNP was found to be superior to that of renal function estimated by the MDRD formula.23
Cystatin C possesses molecular and metabolic characteristics that make plasma concentrations of this protein a good biological marker for estimating renal function; small functional alterations are detected with a higher sensitivity than the conventional parameters used for this purpose.24 This may justify the fact that in the group of patients with serum cystatin C concentrations above the upper normal limit, 15% presented a normal glomerular filtration rate; determination of statin C may be useful for identifying patients with a preclinical state of renal disease.6
The results obtained in the present study are, to a certain degree, in keeping with the reported findings indicating that cystatin C is an independent predictor of cardiovascular complications in patients with coronary disease.25-27 Moreover, our data can extend this concept to include patients with a normal glomerular filtration rate. Jernberg et al26 analyzed the relationship between plasma cystatin C values and prognosis in a group of patients hospitalized for non-ST-segment-elevation ACS, and reported a significant association between cystatin C values and mortality. It has also been described that elevated cystatin C concentrations are associated with an increased risk of death, cardiovascular complications, and the incidence of heart failure in outpatients with chronic coronary disease.24 In older persons without manifest renal disease, cystatin C is a risk marker for death, cardiovascular disease, and chronic renal disease.6 In the current study, we found that patients with elevated cystatin C levels had a poorer risk profile; however, the relatively low C-reactive protein level was surprising. We believe this can be explained by the kinetics of the protein, which has a specific, characterized spectrum in which its value depends on the time point when the sample is drawn (the peak occurs at 49 hours following the onset of symptoms, and a more delayed peak is seen in ST-segment-elevation acute myocardial infarction); in our study, samples were taken within the first 24 hours following hospitalization.28 The cardiovascular complications affecting the patients in our study differ from those published in recent registries,29 and this may be because patients at very high risk and with a higher incidence of complications were included. In the recently published MASCARA registry, only 50% of the patients included had been initially admitted to an ICU or cardiac ICU, whereas all our patients came from a cardiac ICU, a fact that undoubtedly implies some selection bias. Nevertheless, the patient group with elevated cystatin C comprised a population with a significantly higher-risk profile and number of cardiovascular complications than the remaining patients. The present study contributes to establish greater precision in these associations by providing the first report that elevated cystatin C levels in the first hours of hospitalization for high-risk ACS are an independent predictor of in-hospital cardiovascular complications. Moreover, the association between cystatin C and a risk of cardiovascular complications is higher than that of other widely used parameters for estimating renal function, and is maintained even in the group of patients with normal glomerular filtration. Currently, it is not precisely known whether the capability of predicting a higher risk of complications stems from the fact that cystatin C is a better marker of renal function than other common parameters (serum creatinine and glomerular filtration rate), or that there are other factors apart from glomerular filtration that affect the cystatin C concentration and could be directly related to cardiovascular risk. In this line, a positive correlation of plasma C-reactive protein values and fibrinogen with elevated cystatin C concentrations and the presence of cardiovascular disease has been described.30 In the PRIME study (Prospective Epidemiological Study of Myocardial Infarction), the association between cystatin C and development of acute myocardial infarction cardiac death and angina was investigated in patients without coronary disease. After adjusting for the common cardiovascular risk factors, plasma cystatin C level was significantly associated with development of the first coronary complication of ischemic origin. In the opinion of the authors, the decreased glomerular filtration rate would not justify the higher cystatin C values presented by the cases with respect to the controls, and they proposed that inflammation could be at the origin of the relationship between cystatin C and the risk of cardiovascular disease.31,32 In keeping with these data, our results showed a positive correlation between cystatin C and high-sensitivity C-reactive protein (r=0.2), which is also an independent predictor of cardiovascular complications, and provide further evidence to justify the presence of direct relationships between renal disease, inflammation, and cardiovascular disease.
Among the main limitations of the study presented, we should point out that the results refer only to patients with high-risk ACS hospitalized in a cardiac ICU, the majority aggressively treated with early coronary angiography. On the other hand, these characteristics should be highlighted, because there is little information on this specific population, in whom cystatin C was measured at hospital admission. In almost all the published studies, the time point of the determinations is not indicated, and, at least in patients with ACS, plasma cystatin C concentrations can be influenced by the length of time since the event and the diagnostic and therapeutic interventions performed. The limitation of a short follow-up impedes extending our observations to the medium- or long-term. Nonetheless, our findings offer a possibility to improve risk stratification in patients hospitalized for high-risk ACS, which will be useful for deciding therapy as well as establishing the diagnosis.
CONCLUSIONS
Cystatin C determination at the time of hospitalization in patients with high-risk ACS may be a good clinical tool for stratification of cardiovascular risk. Determination of this protein would complement the information provided by other methods of assessing renal function and, in addition to the diagnostic implications, could be useful for identifying the group at highest risk. It may be necessary to pay special attention to fulfilling the recommendations contained in clinical practice guidelines in this population. Moreover, cystatin C could contribute important information for stratifying patients with high-risk ACS and preserved renal function. Additional studies with a longer follow-up may be needed to define the role of cystatin C in ACS with greater precision.
ABBREVIATIONS
ACS: acute coronary syndrome
MDRD: Modification of Diet in Renal Disease
TpI: troponin I
Correspondence:
Dr. J.M. García Acuña.
Servicio de Cardiología y Unidad Coronaria. Travesía da Choupana, s/n. 15706 Santiago de Compostela. A Coruña. España.
E-mail: jose.maria.garcia.acuna@sergas.es
Received April 14, 2008.
Accepted for publication February 10, 2009.