This study aimed to determine the safety and efficacy of modifying the classic implantation technique for aortic transcatheter heart valve (THV) implantation to a cusp-overlap-projection (COP) technique to achieve a higher implantation depth and to reduce the burden of new permanent pacemaker implantation (PPMI) at 30 days. Aortic self-expanding THV carries an elevated risk for PPMI. A higher implantation depth minimizes the damage in the conduction system and may reduce PPMI rates.
MethodsFrom March 2017, 226 patients were consecutively included: 113 patients were treated using the COP implantation technique compared with the previous 113 consecutive patients treated using the classic technique. In all patients, implantation depth was assessed by 3 methods (noncoronary cusp to the THV, mean of the noncoronary cusp and the left coronary cusp to the THV, and the deepest edge from the left coronary cusp and the noncoronary cusp to the THV).
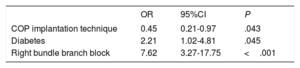
ResultsThe COP group had a lower implantation depth than the group treated with the classic technique (4.8 mm± 2.2 vs 5.7 mm± 3.1; P=.011; 5.8 mm± 3.1 vs 6.5 mm± 2.4; P=.095; 7.1 mm± 2.8 vs 7.4 mm±3.2; P=.392). Forty patients (17.7%) required a new PPMI after the 30-day follow-up but this requirement was significantly lower in the COP group (12.4% vs 23%, P=.036). The COP implantation technique consistently protected against the main event (OR, 0.45; 95%CI, 0.21-0.97; P=.043), with similar procedural success rates and complications.
ConclusionsThe COP implantation technique is a simple modification of the implantation protocol and provides a higher implantation depth of self-expanding-THV with lower conduction disturbances and PPMI rates.
Keywords
During the last few years, transcatheter aortic valve replacement (TAVI) has become the standard of care for severe aortic stenosis (AS) in high-risk and inoperable patients and widespread indications have recently been added for medium- and low-risk patients aged> 65 years.1–3 Several variables have helped to reduce procedural complications and increase success rates: operator/center experience, technical advances in valve designs and delivery systems, and routine preprocedure analysis with multislice computed tomography (MSCT) for accurate sizing of the valves and optimal vascular access. Currently, TAVI is a standard procedure worldwide with a continuing yearly increase in implant numbers.1–3
Despite all these advances, the occurrence of conduction disturbances, such as high degree atrioventricular block (HAVB) and complete heart block requiring new permanent pacemaker implantation (PPMI) is still a significant concern.4 Even with the latest generation of transcatheter heart valves (THV), the PPMI rate has not decreased over time, with a higher incidence with self-expandable THV (17.4%) compared with balloon-expandable THV (6.5%).1 Several factors have been associated with PPMI after TAVI, such as conduction disturbances at baseline, aortic valve predilatation, the use of self-expandable THVs, and implantation depth (ID).4,5
Although ID is one of the most relevant modifiable intraprocedural factors, there is still debate on how to ensure an optimal implantation depth (OID) and obtain an accurate measure of the final THV depth.6 A cusp-overlap projection (COP) implantation technique has been proposed for the deployment of self-expanding THVs because it provides the visualization of the real ID during valve deployment. Moreover, COP offers potential advantages such as eliminating parallax, better visualization of the noncoronary cusp, achieving a true coplanar view and higher implantation of the THV,7,8 and in other studies of balloon-expandable THV it did not increase the risk of valve pop-out.9
This implantation technique has been widely extended in clinical practice; however, little information is available about its impact on the development of conduction disturbances,8,9 particularly in the case of self-expanding aortic THV, with the higher pacemaker implantation rate.
Our study aimed to determine the safety and efficacy of this novel strategy (COP implantation technique) to achieve higher ID and its impact on the burden of new PPMI at 30 days.
METHODSStudy population and designWe conducted a pilot, single-center, observational and prospective study. From 28 March 2017 to 12 November 2020, 419 consecutive patients with severe symptomatic AS were referred by our heart team for TAVI and included in the present investigation. After exclusion, 226 patients were included for analysis. Details of excluded patients can be seen in our flow chart (figure 1). Since 2007, 807 patients underwent TAVI in our institution. All patients referred for aortic valve replacement were carefully evaluated by a multidisciplinary heart team. Patients referred for TAVI are followed up according to guidelines and systematic protocols by experienced interventional cardiologists in a specific TAVI outpatient clinic.10,11 All patients provided informed consent and the study was approved by the local ethics committee.
Considering previous reports suggesting an improvement of OID with the modification of implant projection,7,8 from 1 February 2019, the classic 3-cusp coplanar implantation technique (CIT) for self-expanding THV was modified in our center to a COP implantation technique to optimize ID. Our study took advantage of this modification in routine practice and, for the analysis and clinical investigation, compared 2 groups of consecutive patients: group A, 113 consecutive patients (28 March 2017 to 1 February 2019) with CIT and group B, 113 consecutive patients (2 February 2019 to 12 November 2020) with the COP implantation technique. The flow chart of our study provides an overview of patient distribution (figure 1).
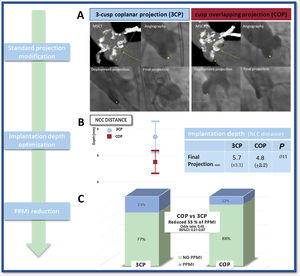
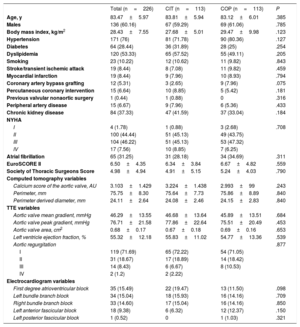
TAVI procedureElectrocardiography (ECG)-gated contrast-enhanced high-resolution MSCT was performed prospectively, according to a systematic protocol, by the same TAVI evaluation team, with experience of more than 900 studies. Image acquisition was performed following standardized recommendations.12 To complete all the specific preprocedural measurements, MSCT data were transferred to a dedicated workstation for evaluation (3Mensio Structural Heart, Pie Medical Imaging BV, The Netherlands) and measurements were performed, including complete assessment of relevant anatomic dimensions and working projections. The COP implantation technique modifies the CIT by overlapping the right coronary cusp and the left coronary cusp (LCC). Cusp-overlap view, as predicted by MSCT, was used for deployment of the device throughout the procedure. The valve size was based on perimeter-derived MSCT measurements with oversizing in borderline cases. The implantation team consisted of 2 interventional cardiologists. The first operator was the same in all the patients, with a wealth of experience with more than 1400 TAVI performed. All procedures were carried out in the cardiac catheterization laboratory under a minimally invasive approach with conscious sedation. The COP implantation technique was similar to CIT in the entire TAVI protocol, except for the deployment projection. A Safari preshaped guidewire (Boston Scientific, USA) was used to position the THV under temporary pacemaker stimulation in the aortic annulus when the prosthesis height was optimal according to the primary operator, the THV was partially released, and angiographic assessment was performed. If the valve hemodynamics were deemed optimal, the prosthesis was implanted. In selected patients, if the risk of coronary occlusion was high (coronary ostium <10 mm of the aortic annulus and sinus of Valsalva height <15 mm for size 23, 26, 29 THV and <16 mm for size 34) a guide catheter engaging the coronary ostium with an intracoronary guidewire was placed in case of acute obstruction. Predilatation was done when valves were heavily calcified and in critical AS with reduced LVEF. COP was identified by MSCT in the preprocedural assessment and subsequently confirmed by intraprocedural angiography during the TAVI procedure (figure 2A).
COP to reduce the new pacemaker implantation rate after TAVI. A: multislice computed tomography and angiography images representing classic implantation technique and cusp overlapping projection technique; B: mean, standard deviation and 95%CI of implantation depth measured from the noncoronary cusp to the prosthesis; C: pacemaker rate according to the classic implantation technique vs the cusp overlapping projection technique. 3CP, 3-cusp coplanar projection; 95%CI, 95% confidence interval; COP, cusp-overlap projection technique; MSCT, multislice computed tomography; NCC, noncoronary cusp; PPMI, permanent pacemaker implantation; TAVI, transcatheter aortic valve replacement.
Primary endpoints were ID and 30-day new pacemaker implantation. Secondary endpoints were the occurrence of HAVB and Valve Academic Research Consortium 2 (VARC-2) safety definitions.
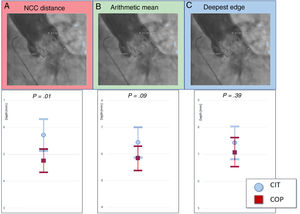
Implantation depth assessmentThe ID was measured angiographically by 2 experienced operators retrospectively using the SyngoDynamics software (Siemens Healthcare, USA). Since there are no standardized definitions in the current VARC-2 criteria on how to assess ID,10 we decided to use 3 of the most common methods used for THVs depth assessment (figure 2A): a) noncoronary cusp (NCC) distance: distance from the deepest portion of the NCC cusp to the distal end of the intraventricular edge of the THV; b) arithmetic mean of the NCC and LCC: the distance from the distal end of the intraventricular portion of the THV to the NCC and the LCC were measured, respectively and the mean was reported; c) deepest edge: the distance from the distal end of the intraventricular portion of the THV to the NCC and the LCC were measured, respectively and only the deepest of the 2 measurements was taken into account.
The ID was measured by angiography using 20mL of Optiray (ioversol) low-osmolality contrast medium at a flow rate of 20mL/s in the final projection in a perpendicular plane to the THV. Interobserver reliability was assessed by randomly measuring ID in 100 patients by 2 physicians from the NCC and the LCC, respectively, to the THV (figure 3A).
Implantation depth. A: measurement from the NCC to the distal end of the intraventricular portion of the prosthesis (2.3mm). B: LCC and NCC distance to the distal end of the intraventricular portion of the valve are measured and the arithmetic mean is reported (2.3+8.2/2=5.25 mm). C: the deepest edge from the LCC and the NCC to the distal end of the prosthesis is reported (2.3 <8.2=8.2 mm). CIT, classic implantation technique; COP, cusp-overlap projection; LCC, left coronary cusp; NCC, noncoronary cusp.
Follow-up of clinical and hemodynamic outcomes was prospectively assessed according to VARC-2 criteria.13 After TAVI, all patients remained under continuous ECG monitoring for at least 48hours. Before discharge and at the 30-day follow-up, 12-lead ECGs were recorded for each patient. A HAVB was defined as the development of second- or third-degree AVB on postprocedural ECG. PPMI was performed if the patient developed HAVB or if a persistent temporary pacemaker was necessary, in accordance with current guidelines recommendations.14–16 After discharge, each patient was followed up at the dedicated TAVI outpatient clinic with a predefined scheduled follow-up with a new ECG. There were no reported losses.
Study variablesStudy variables were included in a specifically dedicated database. Variables related to clinical follow-up were documented following VARC-2 criteria.13
Statistical analysisAnalysis of the normality of the continuous variables was performed with the Kolmogorov Smirnov test. Normally distributed continuous variables are expressed as the mean±standard deviation and were compared using the t-test. Continuous variables with nonnormal distribution are expressed as median values [interquartile intervals] and were compared with the Wilcoxon rank test. Categorical variables are expressed as counts (percentages) and compared using chi-square tests.
Logistic regression analysis was used to test the association between potential risk factors and 30-day PPMI. Variables with P <.1 on univariable analysis were entered into a multivariable model. Associations are expressed as odds ratios (OR) with 95% confidence interval (95%CI). To compare mortality, Kaplan-Meier curves were constructed, and the log-rank test was performed. Differences were considered to be statistically significant if the null hypothesis could be rejected with 95%CI. Statistical analyses were conducted using STATA 14 statistical software package (STATA Corp LP, USA).
The Pearson correlation coefficient was used to assess interobserver reliability for the depth measurements from the LCC and the NCC to the valve in 100 randomly chosen cases and were interpreted as follows:> 0.8, excellent agreement; 0.6 to 0.8, fair to good agreement; 0.4 to 0.6, moderate agreement; and <0.4, no agreement.
RESULTSBaseline characteristicsFrom February 2019, 113 patients were treated using the COP implantation technique and were compared with the previous consecutive 113 patients treated with the CIT (figure 1). The mean age was 83.5±6 years, 136 patients were male (60.2%), there was a similar distribution of cardiovascular risk factors between groups and relevant past medical history. Patients had an intermediate risk for surgery with a mean EuroSCORE II of 6.5 (± 4.4) and a Society of Thoracic Surgeons score of 5 (± 4.9) (table 1).
Baseline characteristics
| Total (n=226) | CIT (n=113) | COP (n=113) | P | |
|---|---|---|---|---|
| Age, y | 83.47±5.97 | 83.81±5.94 | 83.12±6.01 | .385 |
| Males | 136 (60.16) | 67 (59.29) | 69 (61.06) | .785 |
| Body mass index, kg/m2 | 28.43±7.55 | 27.68±5.01 | 29.47±9.98 | .123 |
| Hypertension | 171 (76) | 81 (71.78) | 90 (80.36) | .127 |
| Diabetes | 64 (28.44) | 36 (31.89) | 28 (25) | .254 |
| Dyslipidemia | 120 (53.33) | 65 (57.52) | 55 (49.11) | .205 |
| Smoking | 23 (10.22) | 12 (10.62) | 11 (9.82) | .843 |
| Stroke/transient ischemic attack | 19 (8.44) | 8 (7.08) | 11 (9.82) | .459 |
| Myocardial infarction | 19 (8.44) | 9 (7.96) | 10 (8.93) | .794 |
| Coronary artery bypass grafting | 12 (5.31) | 3 (2.65) | 9 (7.96) | .075 |
| Percutaneous coronary intervention | 15 (6.64) | 10 (8.85) | 5 (5.42) | .181 |
| Previous valvular nonaortic surgery | 1 (0.44) | 1 (0.88) | 0 | .316 |
| Peripheral artery disease | 15 (6.67) | 9 (7.96) | 6 (5.36) | .433 |
| Chronic kidney disease | 84 (37.33) | 47 (41.59) | 37 (33.04) | .184 |
| NYHA | ||||
| I | 4 (1.78) | 1 (0.88) | 3 (2.68) | .708 |
| II | 100 (44.44) | 51 (45.13) | 49 (43.75) | |
| III | 104 (46.22) | 51 (45.13) | 53 (47.32) | |
| IV | 17 (7.56) | 10 (8.85) | 7 (6.25) | |
| Atrial fibrillation | 65 (31.25) | 31 (28.18) | 34 (34.69) | .311 |
| EuroSCORE II | 6.50±4.35 | 6.34±3.84 | 6.67±4.82 | .559 |
| Society of Thoracic Surgeons Score | 4.98±4.94 | 4.91±5.15 | 5.24±4.03 | .790 |
| Computed tomography variables | ||||
| Calcium score of the aortic valve, AU | 3.103±1.429 | 3.224±1.438 | 2.993±99 | .243 |
| Perimeter, mm | 75.75±8.30 | 75.64±7.73 | 75.86±8.89 | .840 |
| Perimeter derived diameter, mm | 24.11±2.64 | 24.08±2.46 | 24.15±2.83 | .840 |
| TTE variables | ||||
| Aortic valve mean gradient, mmHg | 46.29±13.55 | 46.68±13.64 | 45.89±13.51 | .684 |
| Aortic valve peak gradient, mmHg | 76.71±21.58 | 77.86±22.64 | 75.51±20.49 | .453 |
| Aortic valve area, cm2 | 0.68±0.17 | 0.67±0.18 | 0.69±0.16 | .653 |
| Left ventricle ejection fraction, % | 55.32±12.18 | 55.83±11.02 | 54.77±13.36 | .539 |
| Aortic regurgitation | .877 | |||
| I | 119 (71.69) | 65 (72.22) | 54 (71.05) | |
| II | 31 (18.67) | 17 (18.89) | 14 (18.42) | |
| III | 14 (8.43) | 6 (6.67) | 8 (10.53) | |
| IV | 2 (1.2) | 2 (2.22) | ||
| Electrocardiogram variables | ||||
| First degree atrioventricular block | 35 (15.49) | 22 (19.47) | 13 (11.50) | .098 |
| Left bundle branch block | 34 (15.04) | 18 (15.93) | 16 (14.16) | .709 |
| Right bundle branch block | 33 (14.60) | 17 (15.04) | 16 (14.16) | .850 |
| Left anterior fascicular block | 18 (9.38) | 6 (6.32) | 12 (12.37) | .150 |
| Left posterior fascicular block | 1 (0.52) | 0 | 1 (1.03) | .321 |
AU, Agatston units; CIT, classic implantation technique; COP, cusp overlapping projection technique; NYHA, New York Heart Association; TTE, transthoracic echocardiography.
Values are expressed as mean±standard deviation or No. (%).
In the ECG analysis, 121 patients (53.3%) presented previous conduction disturbances, the most frequent were first-degree atrioventricular block in 35 patients (15.5%), left bundle branch block in 34 patients (15%) and right bundle branch block in 33 patients (14.7%). Regarding the echocardiographic findings, the mean gradient was 46.3mmHg (± 13.6) and the peak gradient was 76.7mmHg (± 21.6). The MSCT scan showed a mean perimeter of 75.8mm (± 8.3) and a calcium score of 3102 Agatston units (± 1428). There were no significant differences between groups (table 1).
Procedural data, hospitalization, and 30-day outcomesProcedural success was achieved in 96.5% of the patients. The most frequent vascular access was the right femoral artery in 182 patients (80.5%). Evolut R pro 29 was used in 106 patients (46.9%), predilation was required in 34 (15%), and postdilatation was required in 78 (34.5%). Only 8 patients (3.5%) required a second valve due to severe aortic regurgitation assessed by angiography, there was 1 case (0.4%) of THV embolization in the CIT group, and the mean fluoroscopy time was 27.1minutes±13.4. HAVB appeared in 38 procedures (16.8%) with 25 cases (22.1%) in the CIT group and 13 (11.5%) in the COP group (P=.0328). There were no cases of acute coronary occlusion and no acute coronary syndromes in the first 30 days. Intraprocedural new-onset left bundle branch block was detected in 34 patients (17.7%). Only 1 patient (0.4%) died during the procedure due to a cardiac tamponade secondary to a ventricular laceration in the COP group (table 2).
Procedural data and 30-day outcomes
| Total (n=226) | CIT (n=113) | COP (n=113) | P | |
|---|---|---|---|---|
| Femoral access | .093 | |||
| Right | 182 (80.53) | 86 (76.11) | 96 (84.96) | |
| Left | 44 (19.47) | 27 (23.89) | 17 (15.04) | |
| Predilatation | 34 (15.04) | 21 (18.58) | 13 (11.50) | .137 |
| Postdilatation | 78 (34.51) | 45 (39.82) | 33 (29.20) | .093 |
| Valve size | .329 | |||
| 23 | 3 (1.33) | 3 (2.65) | 0 | |
| 26 | 54 (23.89) | 30 (26.55) | 24 (21.24) | |
| 29 | 106 (46.90) | 49 (43.36) | 57 (50.44) | |
| 34 | 63 (27.88) | 31 (27.43) | 32 (28.32) | |
| Severe final aortic regurgitation | 2 (0.88) | 1 (0.88) | 1 (0.89) | .418 |
| Valve embolization | 1 (0.44) | 1 (0.88) | 0 | .3162 |
| Peak to peak gradient, mmHg | 3.71±4.23 | 3.37±4.89 | 4.03±3.49 | .288 |
| Second valve | 8 (3.54) | 4 (3.54) | 4 (3.54) | 1 |
| Fluoroscopy time, min | 27.07±13.42 | 28.65±15.58 | 25.70±11.09 | .116 |
| Procedural success | 218 (96.46) | 109 (96.46) | 109 (96.46) | 1 |
| Intraprocedural high-degree atrioventricular block | 38 (16.81) | 25 (22.12) | 13 (11.50) | .033 |
| Procedural deaths | 1 (0.44) | 0 | 1 (0.88) | .316 |
| Hospitalization | ||||
| Hospitalization length, d | 7.27±7.40 | 7.06±6.69 | 7.51±8.16 | .668 |
| Vascular complications (VARC-2) | .350 | |||
| Minor | 24 (10.62) | 8 (7.08) | 16 (14.16) | |
| Major | 14 (6.19) | 8 (7.08) | 6 (5.31) | |
| Stroke/transient ischemic attack (mRS) | 19 (8.41) | 8 (7.08) | 11 (9.73) | .472 |
| Grade III or IV aortic regurgitation (TTE) | 13 (5.75) | 6 (5.31) | 7 (6.19) | .264 |
| Final mean gradient (TTE), mmHg | 6.45±2.73 | 6.56±2.67 | 6.35±2.79 | .575 |
| New-onset left bundle branch block | 34 (17.71) | 14 (14.74) | 20 (20.62) | .286 |
| 30-day outcomes | ||||
| Permanent pacemaker implantation | 40 (17.70) | 26 (23.01) | 14 (12.39) | .037 |
| Death at 30 days | 12 (5.31) | 7 (6.19) | 5 (4.42) | .553 |
CIT, classical implantation technique; COP, cusp-overlap projection technique; mRS, modified ranking scale for neurological disability; TTE, transthoracic echocardiography; VARC-2, Valve Academic Research Consortium-2.
Values are represented as mean±standard deviation or No. (%).
The ID assessment showed that patients in the COP group had a higher implant compared with those in the CIT group. However, this difference was only statistically significant when we measured from the NCC to the distal end of the THV with a mean depth of 4.8mm (± 2.2) vs 5.7mm (± 3.1); P=.011, for COP vs CIT cases (table 3, figure 2B). There was a tendency toward a higher implant when using the arithmetic mean from the NCC and the LCC, 5.8mm (± 2.4) vs 6.5mm (± 2.9); P=.095 respectively for COP vs CIT cases and no differences when using the deepest edge, 7.1mm (± 2.8) vs 7.42 (± 3.2); P=.392 (figure 3B). The interclass correlation coefficient from the NCC and the LCC to the THV was 0.915 and 0.867, respectively.
Implantation depth assessment
| Depth measured from the NCC | Arithmetic mean from the NCC and the LCC | Deepest edge | |
|---|---|---|---|
| Classic implantation technique, mm | 5.71±3.07 | 6.45±2.94 | 7.42±3.16 |
| Cusp overlapping projection, mm | 4.77±2.22 | 5.84±2.35 | 7.07±2.79 |
| P | .011 | .095 | .392 |
LCC, left coronary cusp; NCC, noncoronary cusp.
CC depth, depth from the noncoronary cusp to the prosthesis; arithmetic mean, from the noncoronary cusp and the left coronary cusp to the prosthesis; deepest edge, highest distance from the noncoronary cusp or the left coronary cusp to the prosthesis.
Values are expressed as mean±standard deviation.
A total of 40 patients (17.7%) required a new PPMI during the first 30 days, 14 (12.4%) in the COP group and 26 (23%) in the CIT group; P=.0365. In the univariable and multivariable analyses, the COP implantation technique consistently protected against the main event (OR, 0.45; 95%CI, 0.21-0.97; P=.043) (table 4, figure 2C).
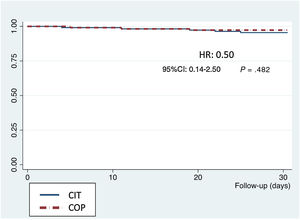
Secondary endpointsIn the 30-day outcomes, there were 12 deaths (table 1 of the supplementary data); 1 of them during the procedure and the remainder during hospital stay (5.3%), as well as 20 strokes/transient ischemic attacks (11.5%), 16 of them (9.2%) with no significant disability according to the modified ranking scale and 4 of them (2.3%) with moderate or higher disability. Only 3 patients (1.6%) had a> grade III paravalvular leak. According to VARC-2 definitions, 14 major (6.2%) and 24 (10.6%) minor vascular access complications occurred, with no differences between the groups (table 2). The Kaplan-Meier survival curves at 30 days showed no significant differences between groups (figure 4). There were no losses to follow-up.
DISCUSSIONThe COP implantation technique has been largely adopted worldwide in TAVI; however, to the best of our knowledge, this is the first study that systematically addresses the safety and efficacy of this technique for self-expanding (SE) aortic valve prosthesis implantation in comparison with CIT. The main findings of our study are as follows: a) this strategy proved to be safe, b) it achieved a higher implant depth and, of most importance, c) reduced the development of atrioventricular conduction disorders and, consequently, the PPMI rate. Our results have straightforward clinical implications. For the first time, we have shown that this change in the implantation procedure significantly reduces one of the most prevalent and feared complications of self-expanding TAVI, the pacemaker implantation rate.
Of utmost importance in the design of this study, we took advantage of 2 consecutive cohorts treated by the same team, under the same protocols and with the same THV. Therefore, this provides a homogeneous sample that overcomes one of the main confusion factors in TAVI trials, namely, the influence of different teams and learning curves.
Our results support that the use of COP implantation technique for SE TAVI is easy and safe, with comparable success rates and similar hemodynamic valve profile as “classic” implantation and it could be potentially used in every case of this SE THV2,17,18. The only procedural difference between the 2 techniques was the modification of the working projection. There were no differences regarding the material used and the steps followed to release the valve. There were low rates of complications such as THV embolization, residual aortic regurgitation, coronary occlusion, and conversion to open-heart surgery. Moreover, there were no differences in 30-day mortality (figure 4) between the 2 groups.
Considering the standardization of MSCT preplanning measurements, the implementation of COP measures should not be difficult in the preprocedural assessment. In our experience, it did not add complexity to the standardized TAVI protocol, as shown in the absence of differences in the fluoroscopy times between the 2 groups.
It is well known that baseline conduction disturbances, such as pre-existing right bundle branch block, first-degree atrioventricular block and left anterior hemiblock, are the most relevant and unmodifiable independent predictors associated with PPMI after TAVI. Other strong predictors, such as predilatation, type of THV and depth of implantation, are modifiable factors and their careful management could reduce the risk of PPMI.4,19 Self-expanding THV has been associated with a 2.5-fold higher risk for PPMI20 and in a current meta-analysis involving the latest generation THV the PPMI rate ranged from 14.7% to 26.7% in self-expanding valves and from 4% to 24% for balloon expandable valves.10 Despite the advances in the design and manufacture of THVs, this problem remains a major unmet clinical need.
The driving idea of our study was how to reduce conduction disturbances related to self-expanding THV? The answer seems clear: performing a higher implant to minimize the damage in the conduction system. However, a higher implant confers a more significant risk of valve embolization and paravalvular leak. An OID is paramount to improve outcomes. Implantation 5 to 7mm below the aortic annulus increases the risk of PPMI, whereas a higher release can reduce the risk. In contrast, ID <3 mm could increase the risk of paravalvular regurgitation and coronary occlusion.21 Accordingly, the manufacturer recommends an OID of 3 to 5mm below the aortic annulus to obtain better results.
This is the main advantage of the COP implantation technique for TAVI because it allows the proper alignment of the sinus by the superposition of 2 of them to perform a higher deployment of the valve, minimizing the risk of valve embolization (figure 2A). In agreement with other studies, our ratio of valve embolization was low, with only 1 case in the CIT group (0.88%) and no cases in the COP technique group (P=.316).9 Moreover, it may offer other potential benefits during the deployment, such as removing the delivery system's parallax and centering it across the aortic annulus. Furthermore, this technique allows maintaining the basal plane alignment of the coronary cusps with a true coplanar view and optimizing the ID because it gives an excellent anatomical reference and a shorter visual distance of the valve and the NCC.8,22
To the best of our knowledge, there is no standardized definition of how to measure the distance from the aortic annulus to the distal end of the THV. Several approaches have been reported to measure this distance, but a reference or standardized method is lacking. Many techniques have been used: the arithmetic mean of the distance from the NCC and the LCC to the THV, the distance from the NCC to the prosthesis, and the deepest edge from the NCC or the LCC to the valve.6
In addition, there is no consensus on the best fluoroscopic projection to choose for the accurate measurement of this distance. The deployment projection23 and the final projection, where the 3 leaflets are aligned and in the same plane,24 have been used.
One of the strengths of our study is the careful and consistent measurement of valve depth. Since there is no consensus on the best way to assess this distance, we decided to perform all the reported measurement methods to date.11–13 Two experimented interventional cardiologists systematically measured all the cases with a high concordance rate using the same practices as those described previously.11–13 ID was numerically higher with the 3 methods in COP implants compared with CIT implants.
Our study concurs with previous studies in terms of showing an independent association of ID with conduction disorders and PPMI, but we demonstrated a significant reduction (OR, 0.45; 95%CI, 0.21-0.97) in their rates with the use of the COP implantation technique for TAVI.
We emphasize the beneficial clinical consequences of the COP TAVI procedure because many of the potentially modifiable factors involved in reducing conduction disturbances after TAVI may not be suitable for modification (eg, predilatation of a heavily calcified valve) and the choice of THV may be subject to the availability of the operating center. However, planning a higher release of the valve could be suitable in most cases.
LimitationsThe main limitations of this study are the modest sample size with a single-center and observational design and that the ID that was retrospectively measured. The primary operator was always the same person and so it could be difficult to extrapolate the data to other operators. Only a multivariable logistic regression analysis was used to predict the main event and, given the nature of the study, some MSCT measurements were not performed, such as the membranous septal anatomy. Finally, our study was focused solely on 1 type of self-expanding THV and therefore the results and conclusions should be interpreted in this context.
CONCLUSIONSOur study suggests that COP implantation technique is a simple modification of the TAVI protocol that provides a higher implantation of self-expanding THV with lower rates of conduction disturbances and PPMI.
- -
Despite all the technical advances in TAVI, the occurrence of conduction disturbances, requiring a new permanent pacemaker implantation, is still a major concern.
- -
In this pilot study, we show for the first time that the adoption of the COP implantation technique for self-expanding aortic valve prostheses significantly reduces the pacemaker implantation rate, one of the most prevalent and feared complications of self-expandable TAVI.
None.
AUTHORS’ CONTRIBUTIONSConceptualization: I. Pascual, P. Avanzas, and C. Morís. Methodology, data curation, investigation, and formal analysis: I. Pascual, M. Almendárez, L. Arboine, R. del Valle, R. Álvarez and D. Hernández-Vaquero. Writing (original draft preparation) I. Pascual and M. Almendárez. Review, editing and supervision: P. Avanzas, F. Alfonso and C. Morís. All authors have read and agreed to the final version of the manuscript.
CONFLICTS OF INTERESTC. Morís is proctor for Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.05.009