Long-term efficacy following cryoballoon ablation of lone paroxysmal atrial fibrillation remains unknown. We describe long-term follow-up results of the single cryoballoon ablation procedure.
MethodsPulmonary vein isolation was performed in 103 patients (72 male; median age 52 years) with symptomatic lone paroxysmal atrial fibrillation. The end-point of this observational cohort study was first electrocardiogram-documented recurrence of arrhythmia (atrial fibrillation, atrial tachycardia, or atrial flutter) during the 5-year follow-up, in the absence of anti-arrhythmic treatment.
ResultsAcute complete pulmonary vein isolation was achieved in 86% of the patients with a single cryoballoon. The 6-month, 1-year, and 5-year success rate after a single procedure was 94%, 91%, and 77%, respectively. Arrhythmia recurrence was observed in 24 cases at a median of 14.8 months [range, 8.0-16.8 months]. Thirteen symptomatic patients were well controlled on beta-blockers only. Seven symptomatic patients had anti-arrhythmic treatment (class IC in 5 patients; dronedarone in 2 patients) introduced during the blanking period. Two of them had early arrhythmia recurrence within the blanking period only; they were arrhythmia-free in further follow-up on dronedarone. The rate of complications was relatively low and included a 4.8% incidence of transient phrenic nerve palsy.
ConclusionsA single cryoballoon ablation procedure for lone paroxysmal atrial fibrillation resulted in high rates of acute, medium-term, and long-term efficacy. The rate of complications is relatively low and includes a 4.8% incidence of transient phrenic nerve palsy.
Keywords
The limited efficacy of drug treatment in patients with atrial fibrillation (AF) is known.1 Catheter ablation of AF is an important therapeutic modality for such patients and the cornerstone of most procedures is pulmonary vein isolation (PVI).2
In recent years, technological improvement led to the implementation of faster and potentially safer “straight-forward” anatomically-oriented methods of PVI. Cryoballoon ablation (CBA) showed their superiority over antiarrhythmic drugs (AAD),3 with a success rate comparable to radiofrequency-based PVI procedures in patients paroxysmal AF.4,5 Recently, high efficacy of CBA as first-line therapy in a selected cohort has also been demonstrated in mid-term follow-up.6
The overall prevalence of AF is 0.4% to 1% in the general population.7,8 Among that group, paroxysmal AF (LPAF)9,10 occurs in 1% of all cases of AF.11 While this population has a relatively low risk of mortality, heart failure, and thromboembolic complications, the risk of progression to persistent or permanent AF was estimated at 47%.11,12 Moreover, the beneficial effects of early abolition of AF to prevent atrial remodeling and evolution to persistent/permanent AF was recently described.13
The latest guidelines14,15 strongly recommend a focus on “low-risk” young patients (< 65 years of age) with AF. The latest consensus underlines the need for reporting long-term success, defined as “freedom from AF, atrial flutter, atrial tachycardia (AFLAT) recurrences, following the 3-month blanking period through a minimum of 36 months follow-up from the date of the ablation procedure in the absence of class I and III AAD therapy”, especially in “newer ablation technologies” such as balloon techniques, and in specific patient populations, such as those with lone AF.2 Following the recommendations,2,14 we present the first very long-term safety and success outcome of CBA in patients with LPAF.
METHODSPatientsPatients with lone AF were defined as young individuals (< 65 years of age) without clinical or echocardiographic evidence of cardiopulmonary disease, including hypertension.9,10,16 Between January 1, 2005 and December 31, 2008, we enrolled 103 consecutive patients with recurrent and drug-refractory LPAF. In all patients, paroxysmal AF was documented by electrocardiogram (ECG) at least once within the last 3 months before ablation. Exclusion criteria were defined as non-paroxysmal AF, any structural heart disease, hypertension, chronic obstructive pulmonary disease treated with beta-sympathomimetic drugs, severe respiratory insufficiency, bleeding diathesis or intolerance of heparin or oral anticoagulation, previous attempted AF ablation, left atrial (LA) thrombus, pregnancy, and severe co-morbidity.
Our single-center study was performed at Kerckoff Klinik, Bad Nauheim, Germany.
Pre-ablationMedical history was obtained during ambulatory visits with a thorough review of the medical records, including ECGs and Holter-ECG recordings showing episodes of AF. All patients gave written informed consent. The study was approved by the local institutional ethics committee. Oral anticoagulation was stopped 3 days before the intervention and replaced by subcutaneous low-molecular-weight heparin. AAD were discontinued at least 3 days before ablation. Beta-blockers were allowed according to the protocol.
All patients underwent echocardiography to determine LA diameter and exclude LA thrombus. The LA size was assessed by measurement of short and long axis in the apical four-chamber view.17
InterventionThe procedure was previously described.18,19 After the transseptal punctures were performed, heparin was introduced with the aim of maintaining activation clotting time >300s throughout the whole procedure. Briefly, we used 23 mm and/or 28 mm cryoballoon (Arctic Front™, Medtronic Cryocath). The single application time was 240 s to 300 s. During CBA of the right-sided pulmonary veins (PVs), unaffected phrenic movement was assessed by continuous phrenic nerve stimulation and continuous monitoring of spontaneous breathing. After initial isolation, recurrence of PV conduction was checked during a 30-min observation period. If PVI could not be achieved with a first-choice cryoballoon size, an additional attempt used a different size. If PVI could not be confirmed after 5 consecutive applications per PV with any balloon, an 8-min tip cryoablation catheter (FreezorMAX™, Medtronic Cryocath) was used for touch-up ablation to complete PVI, which was verified as complete elimination of all PV signals at the antral or ostial level. Additionally, exit and entrance block of all PVs were confirmed by pacing maneuvers, as previously described.18,19
Post-ablation ManagementAfter the procedure, intravenous heparin was continued to achieve a partial thromboplastin time of 60 s to 80 s, followed by oral anticoagulation with coumadin, for at least 3 months, targeting an international normalised ratio of 2-3. Antiarrhythmic treatment was stopped. Beta-blockers were allowed during the follow-up.
Follow-upOur strict follow-up protocol follows the latest recommendations.2 After discharge from the hospital, patients were scheduled for quarterly follow-up visits. Late follow-up (> 1 year post-intervention) was performed annually for 5 years. Seven-day Holter-ECG recordings were obtained at each follow-up visit. Each patient, in case of any palpitations, was instructed to have ECG performed to confirm or exclude AFLAT.
Statistical AnalysisThe study used an observational cohort study design. The end-point of the study was defined as first AFLAT-recurrence after the 3-month blanking period in the 5-year follow-up, in the absence of any AAD. Kaplan-Meier univariate analysis was used to estimate AFLAT-free survival. Continuous data were described median [interquartile range] The discrete variables were given in number and percentage. The differences were considered significant at P<.05.
RESULTSPatients and Procedural CharacteristicsIn total, 103 consecutive patients were enrolled. Clinical and procedural characteristics are displayed in Tables 1 and 2, respectively. Atypical PV anatomy was found in 10 (9.7%) patients: additional right PVs in 2 (1.9%) and common left PV ostium in 8 (7.8%) patients. Acute isolation of all PVs with a single-size cryoballoon was achieved in 85 (82%) patients. A combination of both 23mm and 28mm cryoballoons was applied in 14 (14%) patients and FreezorMax™ was used in 4 (4%) cases. Consequently, complete PVI during the single procedure was achieved in all patients.
Baseline Characteristics
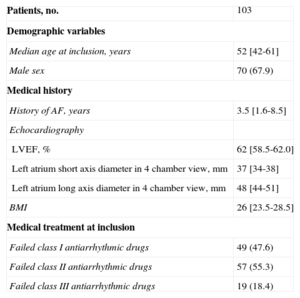
| Patients, no. | 103 |
| Demographic variables | |
| Median age at inclusion, years | 52 [42-61] |
| Male sex | 70 (67.9) |
| Medical history | |
| History of AF, years | 3.5 [1.6-8.5] |
| Echocardiography | |
| LVEF, % | 62 [58.5-62.0] |
| Left atrium short axis diameter in 4 chamber view, mm | 37 [34-38] |
| Left atrium long axis diameter in 4 chamber view, mm | 48 [44-51] |
| BMI | 26 [23.5-28.5] |
| Medical treatment at inclusion | |
| Failed class I antiarrhythmic drugs | 49 (47.6) |
| Failed class II antiarrhythmic drugs | 57 (55.3) |
| Failed class III antiarrhythmic drugs | 19 (18.4) |
AF, atrial fibrillation; BMI, body mass index; LVEF, left ventricular ejection fraction.
Data are expressed as No. (%) or median [interquartile range].
Procedural Characteristics
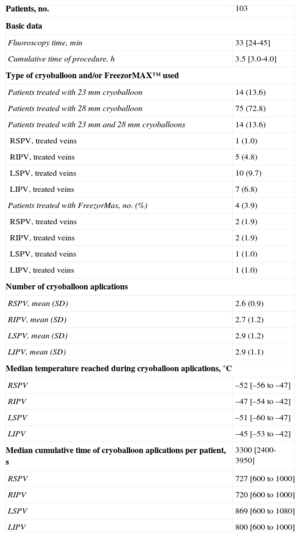
| Patients, no. | 103 |
| Basic data | |
| Fluoroscopy time, min | 33 [24-45] |
| Cumulative time of procedure, h | 3.5 [3.0-4.0] |
| Type of cryoballoon and/or FreezorMAX™ used | |
| Patients treated with 23 mm cryoballoon | 14 (13.6) |
| Patients treated with 28 mm cryoballoon | 75 (72.8) |
| Patients treated with 23 mm and 28 mm cryoballoons | 14 (13.6) |
| RSPV, treated veins | 1 (1.0) |
| RIPV, treated veins | 5 (4.8) |
| LSPV, treated veins | 10 (9.7) |
| LIPV, treated veins | 7 (6.8) |
| Patients treated with FreezorMax, no. (%) | 4 (3.9) |
| RSPV, treated veins | 2 (1.9) |
| RIPV, treated veins | 2 (1.9) |
| LSPV, treated veins | 1 (1.0) |
| LIPV, treated veins | 1 (1.0) |
| Number of cryoballoon aplications | |
| RSPV, mean (SD) | 2.6 (0.9) |
| RIPV, mean (SD) | 2.7 (1.2) |
| LSPV, mean (SD) | 2.9 (1.2) |
| LIPV, mean (SD) | 2.9 (1.1) |
| Median temperature reached during cryoballoon aplications, °C | |
| RSPV | –52 [–56 to –47] |
| RIPV | –47 [–54 to –42] |
| LSPV | –51 [–60 to –47] |
| LIPV | –45 [–53 to –42] |
| Median cumulative time of cryoballoon aplications per patient, s | 3300 [2400-3950] |
| RSPV | 727 [600 to 1000] |
| RIPV | 720 [600 to 1000] |
| LSPV | 869 [600 to 1080] |
| LIPV | 800 [600 to 1000] |
LIPV, left inferior pulmonary vein; LSPV, left superior pulmonary vein; RIPV, right inferior pulmonary vein; RSPV, right superior pulmonary vein; SD, standard deviation.
Data are expressed as No. (%), mean (standard deviation) or median [interquartile range].
Periprocedural complications, with the need for intervention, occurred in 6 patients. One cardiac tamponade was treated with pericardial drainage. In 5 cases, phrenic nerve palsy was noted during right superior PV ablation. Additionally, one pericardial effusion, without the need for intervention, was found. No other complications were observed.
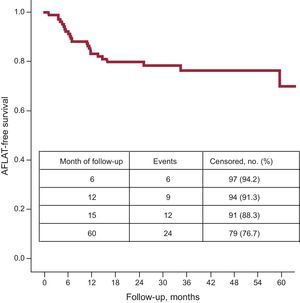
Follow-upNo patient was lost to follow-up. The 5-year success rate, regarding the primary endpoint, after a single CBA procedure was 77% (Figure).
Five-year outcome in patients with lone paroxysmal atrial fibrillation. Kaplan-Meier event-free survival curve after a single cryoballoon ablation procedure in young patients with lone paroxysmal atrial fibrillation. Study group (n = 103). AFLAT, atrial fibrillation, atrial flutter, atrial tachycardia.
Neither the use of AAD before CBA nor the use of an additional cryoballon size and/or FreezorMax™ during the procedure influenced the outcome. Beta-blockers were used in 60 patients (58%); 57 of these patients were already treated with beta-blockers before the CBA procedure. In 3 cases, beta-blockers were introduced during the follow-up. AFLAT recurrence was observed in 24 patients at a median 14.8 months (8.0-16.8 months). Thirteen symptomatic patients were well controlled on beta-blockers only. Seven symptomatic patients had AAD treatment (class IC in 5 patients; dronedarone in 2 patients) introduced during the blanking period, including 1 patient already on beta-blockers. Two of them had early AFLAT recurrence within the blanking period only; they were AFLAT-free in further follow-up on dronedarone. Finally, 7 patients with AFLAT recurrence required a repeat procedure.
DISCUSSIONBoth the latest guidelines14 and consensus statement2 underline the need for reporting long-term success with the use of newer ablation technologies in specific patient populations. Herein, we report very long-term follow-up to date of CBA in LPAF patients.
The overall freedom from AFLAT, after a single CBA procedure and without AAD treatment (except for beta-blockers) during follow-up, was observed in 88% and 77% of patients at 15-month and 5-year follow-up, respectively. The rate of complications was acceptably low.
Rationale for Selection of PatientsAtrial fibrillation is associated with a 5-fold risk of stroke.14 Diagnosing AF before the first complications occur is crucial for the prevention of strokes. Opportunistic screening for AF is recommended in patients ≥ 65 years of age.14 Recent guidelines have strengthened the use of the risk-factor approach to stroke risk stratification. A practice shift toward greater focus on identification of “truly low-risk” patients with AF (ie, age < 65 years and lone AF) has been recommended.14 Moreover, recently adopted CHA2DS2-VASc (congestive heart failure, hypertension, age ≥ 75 [doubled], diabetes, stroke [doubled]-vascular disease and sex category [female]) risk score identified age > 65 years as one of the risk factors.14
A recent international consensus on nomenclature and classification of AF mentioned that only AF in the absence of heart disease should be termed ‘lone’.20,21 The diagnosis of lone AF is essentially a diagnosis of exclusion, and should be preceded by careful evaluation, including a thorough collection of the patient's medical history, physical examination, blood pressure measurement, laboratory tests, ECG, echocardiography, and according to some experts, chest X-ray and exercise testing.15,20 Clinically, 5 types of AF have been distinguished, based on the duration of the arrhythmia: first diagnosed, paroxysmal, persistent, long-standing persistent, and permanent AF.22
Consequently, we focused on truly low-risk young patients with LPAF. The characteristic male preponderance in such a cohort, with a male-to-female ratio of 3:1, was previously described.23
Acute SuccessPrevious systematic review showed that the acute procedural success could be achieved in 99% (range, 92%-100%) of 924 patients.24 Ablation with the cryoballoon catheter alone allowed for PVI in 78% of patients while a hybrid approach, with concomitant focal ablation, achieved complete PVI in 99% of patients.24 In our study, complete isolation was achieved in 96% (99 of 103) of cases with the cryoballoon technique only. Still, we had to change balloon size during 14 procedures in individuals with adverse anatomic conditions. Additional FreezorMax™ lesions, performed in 4 patients, yielded a 100% complete PVI. Comparable results were recently reported by Namdar et al6 in a small cohort of 18 patients with LPAF. In 2 patients, additional radiofrequency-based touch-up lesions were also needed.6
OutcomeThe data on long-term outcome after CBA procedure in young patients with LPAF are missing. The only published report, describing 1-year outcome in patients with LPAF, showed an 82% success rate.6 Still, the major limitation of that study was the low number of patients: only 11 reached the 12-month follow-up.
Our results are promising. After a single CBA procedure, we observed very high overall AFLAT-free survival without AAD treatment: 91% at 12 months, 88% at 15 months and 77% at the 5-year follow-up. Moreover, the total clinical outcome, after including 17 patients with good rhythm control on AAD, was 93% at 5-year follow-up. Only 7 patients needed repeated procedure to relieve symptoms of AF. Progression to persistent AF was not observed in any of the patients.
There are many possible reasons that the studied cohort of young patients with LPAF may have such a good outcome. These patients have nonenlarged atria and paroxysmal AF. In such settings, the probability of a deep remodeling process is low and the development of AF is trigger- and rather than substrate-dependent. Most triggers originate at the LA-PV junction.25 PVI-focused CBA, which eliminates triggers, and partially the substrate, located near the LA-PV junction, results in a high success rate in such patients. Failure of PVI in this group is a result of incomplete isolation of LA-PV triggers or existing non-LA-PV triggers. Moreover, the combination of normal-sized LA and a relatively large balloon may result not only in ostial circumferential PVI but also in PVI by ipsilateral antral block ablation, which might further improve the clinical outcome.26
When making decisions in the young patient regarding AAD vs ablative therapy, factors such as the potential length of time of AAD exposure should be considered.27 Pro-arrhythmic effect of AAD, including lethal ventricular arrhythmias, is well described.28 The duration of time on a certain medication, most notably amiodarone, is associated with further well-known complications.28 Nevertheless, medical treatment has limited efficiency in preserving sinus rhythm.29 The recently published STOP AF trial demonstrated that CBA was more effective than AAD for the treatment of patients with symptomatic paroxysmal AF.3 However, the results of the STOP AF trial cannot be simply extrapolated to our cohort of patients.
Moreover, taking into account the risk of periprocedural silent cerebral ischemia, cryoballoon technology appeared to be one of the safest for the treatment of patients with AF. The incidence of cerebral microembolic signals during catheter ablation of AF was the lowest among patients treated with CBA, compared to radiofrequency catheter.30 Furthermore, the incidence of subclinical intracranial embolic events was lower after CBA (4%), compared to radiofrequency (7%) and multielectrode phased radiofrequency procedures (37%).30 Consequently, the studied population of young individuals with LPAF seems to be best suited for a balloon-based strategy where the goal of intervention is PVI only.
SafetyThe most common complication of the CBA procedure is phrenic nerve palsy, with an overall incidence of 6%.24 Previously reported incidence of phrenic nerve palsy persisting after the ablation procedure was 5% (67 of 1349), with complete recovery within 1 year in most of the patients (62 of 1349).24
We observed 5 (4.8%) cases of phrenic nerve palsy during CBA in right superior PV, none in other PVs. In 4 of these patients, a 28 mm cryoballoon was used. Despite immediate termination of the freezing cycle at loss of phrenic nerve capture, phrenic nerve palsy persisted after the procedure but full recovery was observed in all 5 patients within 12 months.
A recent report showed that the systematic use of phrenic nerve stimulation in conjunction with a 28 mm cryoballoon catheter could not eliminate the problem of phrenic nerve palsy.31 The anatomical relationship between right superior PV and superior vena cava was proposed as a predictor for the development of phrenic nerve palsy during CBA with a 28 mm cryoballoon catheter: predictors, prevention, and low cryoballoon temperature early in the freezing cycle provided a sensitive warning sign of impending phrenic nerve palsy.32 Preprocedural imaging of the right superior PV and superior vena cava relationship, avoiding the antral catheter position in right superior PV, close monitoring of temperature and abrupt cessation of energy delivery,32 or immediate balloon deflation33 may further enhance the safety of the procedure. Recently, monitoring the amplitude of the diaphragmatic compound motor action potentials has been proposed for the detection of impending hemidiaphragmatic paralysis.34
Study LimitationsThis study has the following potential limitations: a) this is a single-center, non-randomized, prospective report with the inherent limitations of this study design. However, there was no selection bias for study inclusion because all consecutive patients undergoing CBA procedure for LPAF at our institution were included for analysis; b) according to the latest consensus,2 the follow-up results are presented without consideration of recurrences during the blanking period. This could lead to an overestimation of the true success rate, and c) the most recent data,35,36 unknown at the time of our study, show that intermittent rhythm monitoring is significantly inferior to continuous monitoring with implanted devices. Our follow-up was based on clinical evaluation and 7-day Holter ECG recordings. Although the vast majority of patients presented with sustained forms of AFLAT, some asymptomatic nonsustained episodes may have been missed. Any missed asymptomatic arrhythmia should have occurred equally across all subgroups and is not likely to have changed the conclusions of our study.4 We did not perform any esophageal investigations to exclude any possible lesions after a procedure. Recently, a high incidence of esophageal lesions using new CBA devices was reported.37–39 Still, the data were unknown at the time of our study and we used only first-generation cryoballoons.
Clinical ImplicationsClinical implications of our results are substantial with regard to the care of young patients with LPAF. First, long-term follow-up data should be presented. Patients with LPAF appear to be the first-line candidates for the CBA procedure. Still, they should be aware of possible procedure-related complications, including phrenic nerve palsy. Further preprocedural imaging and more intense monitoring of phrenic nerve function could minimize the risk of phrenic nerve palsy. Secondly, ongoing surveillance is warranted, even if CBA was deemed initially successful. Incidence of late recurrence may be related to the extent of ECG monitoring and earlier recurrence may be missed in selected patients with no or minimal symptoms.2,36
CONCLUSIONSThis study reveals that a single CBA procedure for LPAF results in high acute, medium- and long-term efficacy rates. The rate of complications is relatively low and includes a 4,8% incidence of transient phrenic nerve palsy.
CONFLICTS OF INTERESTDr. Maciej Wójcik was supported by European Heart Rhythm Association (2-years clinical electrophysiology fellowship, 2007-2009); Prof. Thomas Neumann received speakers’ honoraria from Medtronic Cryocath; Dr. Malte Kuniss and Dr. Heinz-Friedrich Pitschner received speakers’ honoraria and honoraria for advisory board meetings from Medtronic Cryocath.