Adequate, updated and functional technology is essential in cardiology. In Spain, the economic scenario has strongly impacted technology renewal programs and obsolescence is a growing problem. The current report attempts to describe the current situation and the conditions that must concur to update, replace or adopt new technologies in the field of cardiology.
Keywords
Few specialties depend on technology for patient diagnosis and treatment as much as cardiology. Modern cardiology practice relies on the availability of the appropriate technology and its correct operation and functioning. The current economic situation has had a strong impact on technology renewal programs and obsolescence is a growing problem in the European health sector, as indicated by the latest reports available on this topic.1,2
The current report of the Executive Committee of the Spanish Society of Cardiology attempts to describe the current situation and the conditions that must align for technologies in the cardiology field to be updated, replaced, or adopted. The ultimate aim is to uphold quality of care, improve efficiency, maintain the safety of medical activity, and facilitate innovation in a setting of health system sustainability, based on evidence-based criteria that provide objective elements for decision-making.
FUNDAMENTALSPrepared by the Spanish Agency of Medicines and Medical Devices (AEMPS), Circular No. 3/2012 makes recommendations on technical assistance for medical devices in health centers3 based on European legislation (Directives 90/385/CEE,4 93/42/CEE,5 and 98/79/CE6). The memorandum establishes that “medical devices must not compromise the health or safety of patients, users, or third parties when properly installed and maintained and must be used according to their intended purpose” and that “the health authorities of the Member States must adopt the necessary measures and carry out the appropriate monitoring to ensure that these guidelines are effectively met for the products marketed, placed in service, installed, maintained, and used in their territories”. As for equipment maintenance, the circular states that “the products must be correctly installed and properly maintained in such a way as to guarantee that, during their period of use, they maintain the safety and performance intended by their manufacturer“.
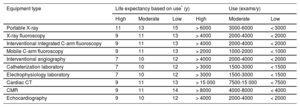
In this regard, the Canadian Association of Radiologists has issued recommendations on the useful life of imaging equipment based on a comprehensive review of the available literature.7 A clinical use exceeding 15 years is not generally recommended for any technology. The association also estimated equipment durability according to its degree of use, categorized as high (24hours 5 d/wk or 750 8-hour shifts/y), medium (16hours 5 d/wk or 500 8-hour shifts/y), and low (8hours 5 d/wk or 250 8-hour shifts/y). Finally, the authors note that ultrasound equipment can experience an accelerated obsolescence because optimal technological upgrades are essential to maintain high diagnostic performance. The expert committee of the Spanish Society of Cardiology (SEC) has similarly established longevity recommendations for equipment used in cardiological diagnostic procedures based on their degree of use (Table 1).
Expected Lifespan of Radiology Equipment According to Use
| Equipment type | Life expectancy based on use* (y) | Use (exams/y) | ||||
|---|---|---|---|---|---|---|
| High | Moderate | Low | High | Moderate | Low | |
| Portable X-ray | 11 | 13 | 15 | > 6000 | 3000-6000 | < 3000 |
| X-ray fluoroscopy | 9 | 11 | 13 | > 4000 | 2000-4000 | < 2000 |
| Interventional integrated C-arm fluoroscopy | 9 | 11 | 13 | > 4000 | 2000-4000 | < 2000 |
| Mobile C-arm fluoroscopy | 9 | 11 | 13 | > 2000 | 1000-2000 | < 1000 |
| Interventional angiography | 7 | 10 | 12 | > 4000 | 2000-4000 | < 2000 |
| Catheterization laboratory | 7 | 10 | 12 | > 3000 | 1500-3000 | < 1500 |
| Electrophysiology laboratory | 7 | 10 | 12 | > 3000 | 1500-3000 | < 1500 |
| Cardiac CT | 9 | 11 | 13 | > 15 000 | 7500-15 000 | < 7500 |
| CMR | 9 | 11 | 14 | > 8000 | 4000-8000 | < 4000 |
| Echocardiography | 9 | 10 | 12 | > 4000 | 2000-4000 | < 2000 |
CMR, cardiac magnetic resonance; CT, computed tomography.
On the other hand, the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR), representing the medical technology industry in Europe, recommends adherence to its “Golden Rules“for the optimal functioning of medical equipment.1 These rules advise the following:
- •
At least 60% of the installed equipment should be less than 5 years old: these systems adequately reflect the current state of the technology.
- •
No more than 30% of the installed equipment should be between 6 and 10 years old: although these systems are still suitable for their purpose, their replacement should be considered.
- •
No more than 10% of the installed equipment should be more than 10 years old: this medical technology is outdated and difficult to maintain and repair.
In addition, the committee makes some general recommendations:
- •
Replace obsolescent equipment that cannot be upgraded.
- •
Use new funding models with the support of the European Fund for Strategic Investments to convert investment in technological innovation in health into a strategy to increase the efficiency and accessibility of health systems and improve clinical outcomes.
- •
Adopt a patient-centered approach to reduce and optimize radiation doses.
The Spanish Society of Cardiology supports these recommendations and believes that their adoption would guarantee the correct functioning of the systems installed, their appropriate use, quality of care, treatment suitability, and patient safety.
CARDIAC IMAGINGEchocardiographyImportance and Current Technological Situation in SpainBecause echocardiography is an easily accessible, safe, and examiner-dependent imaging technique, innovations in both hardware and software are pivotal and the renewal recommendations are thus reduced to 5 to 7 years. This recommendation is vitally important in the case of echocardiography, whose widespread availability in recent years has made it an essential test for the management of cardiac patients, with a corresponding exponential increase in its indication.
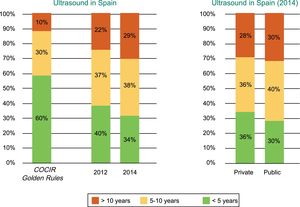
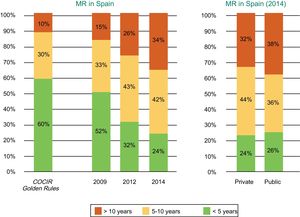
Spain is far from meeting the Golden Rules standards regarding equipment renewal, and 1 out of every 3 ultrasound scanners is obsolete, with an age exceeding 10 years (Figure 1).2
Age of the echocardiography equipment installed in Spain according to year (2012 vs 2014) and whether the center is public or private. COCIR, European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry. Reproduced with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
The scant maintenance of ultrasound equipment is also troubling. More than two-thirds do not undergo preventive maintenance procedures guaranteeing their functioning and calibration and, therefore, image quality and accuracy.2,8
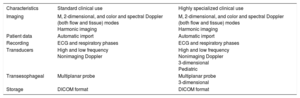
Technological Resource RecommendationsIn 2011, the American Society of Echocardiography issued quality recommendations for echocardiography laboratories.9 First, laboratories must be accredited by the Intersocietal Commission for the Accreditation of Echocardiography Laboratories. Echocardiography systems must be able to obtain images in M, 2-dimensional, and color and spectral Doppler (both flow and tissue) modes. They must be able to add patient identity data to the study and record both the electrocardiogram (ECG) and the respiratory phases. For transthoracic studies, high- and low-frequency transducers should be available, as well as nonimaging Doppler transducers, although these machines are rarely used in clinical practice. Dedicated transducers should be available for pediatric studies. Transesophageal echocardiography should be performed with multiplanar probes.10 Recent years have seen considerable technological advances in 3-dimensional echocardiography. This tool is useful in transthoracic studies to calculate ventricular volume and function and, particularly, in transesophageal studies to obtain a detailed visualization of valvular anatomy and provide important information for the guidance of structural interventions.11 Its performance requires adequate transthoracic and transesophageal matrix transducers. Although this technique is not essential in routine clinical practice, it is recommended in centers performing transcatheter structural interventional cardiology.12 Finally, the systems must have harmonic imaging capabilities and other instrument settings allowing optimization of both standard and contrast-enhanced images. In the case of stress studies, the machine must also allow comparison of the images acquired in the different phases.
Diagnostic images should be stored in DICOM (Digital Imaging and Communications in Medicine) format for as long as specified for later review.
The accuracy of echocardiography equipment should be periodically assessed and the manufacturer's recommendations for preventive maintenance should be followed. Similarly, details on these tests must be recorded and stored.
Thus, evaluation of the potential value of each technology according to use would help to ensure quality of care (Table 2).
Echocardiography Equipment Requirements According to Use
| Characteristics | Standard clinical use | Highly specialized clinical use |
|---|---|---|
| Imaging | M, 2-dimensional, and color and spectral Doppler (both flow and tissue) modes Harmonic imaging | M, 2-dimensional, and color and spectral Doppler (both flow and tissue) modes Harmonic imaging |
| Patient data | Automatic import | Automatic import |
| Recording | ECG and respiratory phases | ECG and respiratory phases |
| Transducers | High and low frequency Nonimaging Doppler | High and low frequency Nonimaging Doppler 3-dimensional Pediatric |
| Transesophageal | Multiplanar probe | Multiplanar probe 3-dimensional |
| Storage | DICOM format | DICOM format |
DICOM, Digital Imaging and Communications in Medicine; ECG, electrocardiography.
Computed tomography (CT), as an imaging technique involving ionizing radiation, is undergoing continuous technological development aimed at improving image quality and reducing patient exposure. In the last 30 years, the scan time has been significantly reduced by increasing the gantry rotation speed and number of detectors, as well as through new acquisition protocols.
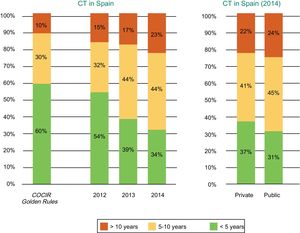
Current Technological Situation in SpainMeasures to contain health spending, a result of the challenging economic situation in Spain, have resulted in a progressive obsolescence of CT equipment in recent years. Both public and private centers have been affected according to data from the Spanish Federation of Healthcare Technology Companies (Fenin) (Figure 2).2
Age of the CT equipment installed in Spain according to year (2012-2014) and whether the center is public or private. COCIR, European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry; CT, computed tomography. Reproduced with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
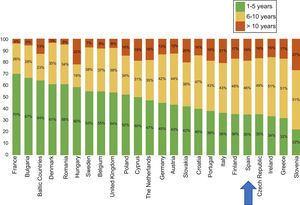
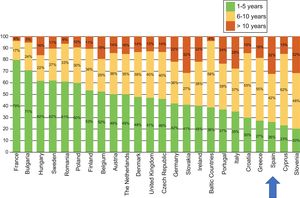
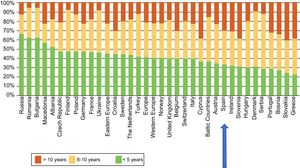
The latest COCIR report of 20161 revealed a continuous increase in the average age of scanners installed in Western Europe, with an increase in the percentage of equipment more than 5 years old from 40% in 2008 to 53% in 2015. These data are especially concerning in Spain, which persistently shows one of the worst performances in terms of adherence to the Golden Rules (Figure 3). Consequently, most equipment in Spain cannot be considered “low dose“, with the harmful effects that this represents for patient safety.
Degree of compliance by country with the Golden Rules of the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR). Adapted with permission from the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry.1
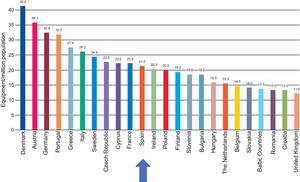
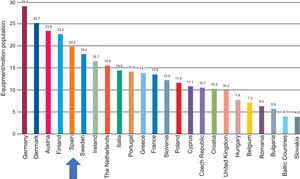
With regard to the number of systems, although the density in Europe has also been reduced, Spain is in an intermediate position, with an average of 21.5 systems per million population. Thus, the recommended minimum of 20 systems per million population is exceeded, albeit only barely (Figure 4).
Computed tomography equipment density by country. Adapted with permission from the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry.1
The Society of Cardiovascular Computed Tomography recently updated their previous 2009 Guidelines for the Performance and Acquisition of Coronary Computed Tomographic Angiography,13 which considers the latest technological advances in this diagnostic tool.
In this update of the guidelines, the society recommends that machines performing tests comply with the accreditation standards published by the ICACTL (Intersocietal Commission for the Accreditation of Computed Tomography Laboratories) or the ACR (American College of Radiology). Computed tomography scanners should have fast gantry rotation (≤ 350ms) for use in cardiac studies. Similarly, the equipment should have at least 64 detectors (collimations of 32×2 or 64×1), with detector element widths of ≤ 0.625 mm. Although the use of single-head injection pumps is considered sufficient, 2 heads are desirable to allow the performance of high-speed biphasic or triphasic injections.
Regarding radiological protection, the “as low as reasonably achievable” (ALARA) principle must be strictly followed. This approach aims to minimize the radiation dose without affecting the diagnostic usefulness of the images obtained. Some of the factors determining the dose received by patients are easily modifiable in all CT systems (eg, tube voltage and current, scan area, slice thickness). However, many other factors, including the source type (single vs dual), acquisition time, gantry rotation speed, acquisition type (prospective, retrospective, high-pitch helical), dose modulation, and reconstruction method (filtered back projection vs iterative reconstruction), are only modifiable in new-generation equipment. The 2016 COCIR report1 places special emphasis on the use of dose-modulation techniques and iterative reconstruction algorithms, which can reduce the radiation absorbed by up to 82%.
In this regard, a quarter of the CT systems installed in Europe are too old to be updated with these technologies, and Spain is one of the European countries with the highest proportion of these systems. Another fundamental aspect in radiological control is the performance of mandatory maintenance protocols. Despite supervision by accredited radiologists, the Fenin report8 estimates that up to 31% of machines do not receive preventive maintenance to certify their functionality. Finally, information on each patient's radiation exposure must be stored in a format permitting later review and evaluation.
Images must be stored in DICOM format and a PACS (Picture Archiving and Communication System) or similar system must be available to allow the storage and recovery of all diagnostic images.
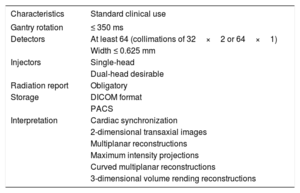
Finally, for the analysis and interpretation of cardiac CT, specific software platforms must be used to evaluate 3-dimensional images with cardiac synchronization obtained in all conventional reconstruction formats: 2-dimensional transaxial images, multiplanar reconstructions, maximum intensity projections, curved multiplanar reconstructions, and 3-dimensional volume rendering reconstructions.14
Thus, evaluation of the potential value and essential elements of each technology would help to ensure quality of care (Table 3).
Cardiac Computed Tomography Requirements
| Characteristics | Standard clinical use |
|---|---|
| Gantry rotation | ≤ 350 ms |
| Detectors | At least 64 (collimations of 32×2 or 64×1) |
| Width ≤ 0.625 mm | |
| Injectors | Single-head |
| Dual-head desirable | |
| Radiation report | Obligatory |
| Storage | DICOM format |
| PACS | |
| Interpretation | Cardiac synchronization |
| 2-dimensional transaxial images | |
| Multiplanar reconstructions | |
| Maximum intensity projections | |
| Curved multiplanar reconstructions | |
| 3-dimensional volume rending reconstructions |
DICOM, Digital Imaging and Communications in Medicine; PACS, Picture Archiving and Communication System.
Cardiac magnetic resonance (CMR) imaging is a noninvasive imaging technique that does not use ionizing radiation but allows tissue characterization and a detailed evaluation of cardiac anatomy and function without acoustic window limitations. Accordingly, it has been established for many years as a highly useful diagnostic tool in routine clinical practice.15
Current Technological Situation in SpainThe 2016 COCIR study1 shows that, although the age profile of the magnetic resonance equipment has not deteriorated vs the previous evaluation, it still does not meet the recommendations of the Golden Rules. As in the case of CT, Spain is in one of the last positions, with a large percentage of machines more than 5 years old, and its situation has even slightly worsened vs previous registries (Figure 5 and Figure 6).
Age of the magnetic resonance equipment installed in Spain according to year (2009, 2012, and 2014) and whether the center is public or private. COCIR, European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry; MR, magnetic resonance. Reproduced with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
Degree of compliance with the Golden Rules of the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry (COCIR) in magnetic resonance. Adapted with permission from the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry.1
In terms of magnetic resonance equipment density, Spain is in a good position vs other European countries and complies with the guideline of more than 20 devices per million population (Figure 7).
Magnetic resonance equipment density by country. Adapted with permission from the European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry.1
The advantage of this imaging technique is that, by modifying other hardware elements and the software, relevant updates can be made without affecting the most important parts of the equipment (the magnet and Faraday cage isolation system). This permits somewhat older machines to meet current diagnostic standards. However, the manufacturer or an authorized representative handles the maintenance of this equipment in only 83% of cases2 and the preventive maintenance is limited to 77% of resonance systems.8 Thus, a significant percentage of machines may not benefit from the available updates.
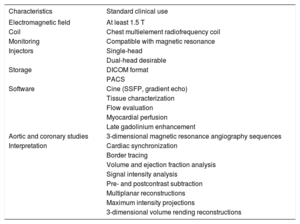
Technological Resource RecommendationsA magnetic resonance device with an electromagnetic field of at least 1.5 T is required for CMR.16 Although 3-T systems have an improved signal-to-noise ratio that allows better evaluation of first-pass perfusion sequences, as well as tagging sequences and 4-dimensional flow techniques, they are more susceptible to artifacts, which may hinder the acquisition of SSFP sequences. Thus, although both fields can be used for CMR studies, 1.5-T systems continue to be the standard. Other necessary hardware elements are a chest multielement radiofrequency coil and an ECG monitoring system compatible with magnetic resonance. As for CT, these systems will ideally have a dual-head injector that allows for high-speed injection. The most important aspect for CMR studies is that the equipment has the necessary software package. It must consist of at least the sequences necessary for clinical studies: cine (SSFP, gradient echocardiography), tissue characterization (T1- and T2-weighted sequences, T2* sequences), flow evaluation (phase-contrast sequences), myocardial perfusion (saturation-recovery sequences), and late gadolinium enhancement (T1-weighted inversion-recovery sequences). In addition, aortic and coronary studies require 3-dimensional magnetic resonance angiography sequences with or without contrast; the latter require a navigation system. Therefore, magnetic resonance equipment destined for cardiac studies must have all of these sequences or be physically able to undergo the required upgrade. Recent times have shown considerable development of new techniques for evaluating the myocardial interstitial space through T1 and T2 mapping.17 These new tools require specific sequences, protocols, and analysis software,18 with technological requirements not available in older resonance equipment. However, these protocols are currently not an integral part of the usual clinical studies. Therefore, although their development is desirable, their availability is not yet mandatory for routine clinical practice.
In the same way as with other cardiac imaging techniques, the data obtained should be stored in DICOM format for subsequent analysis and revision.
According to the Society for Cardiovascular Magnetic Resonance,19 the software and hardware used for CMR image analysis must meet certain minimum requirements. A workstation and screen with adequate specifications and sufficient resolution are required. The analysis software should allow the simultaneous visualization of all short axes and the tracing of the endocardial and epicardial borders, as well as the selection of the most basal segment for ventricular volume and function analysis. Cross-references must be possible to confirm slice position. It must be possible to simultaneously compare the same slice between different sequences, long- and short-axis images, and different studies of the same patient. Semiquantitative analysis of signal intensity must also be possible. In the case of vascular studies, it must be possible to subtract postcontrast from precontrast datasets, as well as to perform multiplanar, maximum intensity, and 3-dimensional volume rendering reconstructions.
Therefore, evaluation of the potential value and essential elements of each technology would help to ensure quality of care (Table 4).
Magnetic Resonance Equipment Requirements for Diagnostic Cardiology
| Characteristics | Standard clinical use |
|---|---|
| Electromagnetic field | At least 1.5 T |
| Coil | Chest multielement radiofrequency coil |
| Monitoring | Compatible with magnetic resonance |
| Injectors | Single-head |
| Dual-head desirable | |
| Storage | DICOM format |
| PACS | |
| Software | Cine (SSFP, gradient echo) |
| Tissue characterization | |
| Flow evaluation | |
| Myocardial perfusion | |
| Late gadolinium enhancement | |
| Aortic and coronary studies | 3-dimensional magnetic resonance angiography sequences |
| Interpretation | Cardiac synchronization |
| Border tracing | |
| Volume and ejection fraction analysis | |
| Signal intensity analysis | |
| Pre- and postcontrast subtraction | |
| Multiplanar reconstructions | |
| Maximum intensity projections | |
| 3-dimensional volume rending reconstructions |
DICOM, Digital Imaging and Communications in Medicine; PACS, Picture Archiving and Communication System; SSFP, Steady-state Free Precession.
Images in catheterization laboratories are generally obtained using X-ray equipment. This technology generates ionizing radiation, is subject to robust safety-related regulation, and must comply with European safety regulations, Directive 2013/59/EURATOM,20 which will be transposed and applicable from April 2018.
Equipment for the Percutaneous Treatment of Coronary LesionsPercutaneous treatment of coronary lesions currently represents the main activity of catheterization laboratories. The management of such lesions requires radiological equipment capable of generating images of sufficient quality to ensure intervention success.
In addition to equipment providing optimal image quality, additional intracoronary diagnostic techniques should be available:
- •
Fractional flow reserve (FFR): currently the technique of choice for analysis of the functional severity of angiographically intermediate coronary lesions because it avoids unnecessary revascularization in insignificant lesions from a physiological point of view. Clinical practice guidelines recommend the use of this technique to identify hemodynamically relevant lesions in stable patients (class I indication, level of evidence A).21 Accordingly, this technique should be available in all catheterization laboratories.
- •
Intravascular ultrasound (IVUS) and optical coherence tomography: both imaging techniques permit detailed evaluation of coronary lesion morphology, as well as outcome optimization and analysis of outcomes after stent implantation. Optical coherence tomography has better spatial resolution than IVUS and allows a more accurate detection of intraluminal structures. It is also superior to IVUS for assessment of stent malapposition, thrombus or plaque prolapse, and dissections and for evaluation of stent failure mechanisms. According to myocardial revascularization guidelines,21 both techniques are useful for studying the mechanisms of stent failure (IIa B) and optimizing stent implantation (IIa B for IVUS and IIb C for optical coherence tomography). In addition, IVUS is recommended to study the severity of left main coronary artery lesions and optimize outcomes (IIa B). Some of these systems should be available for the management of complex injuries.
Compared with the treatment of coronary disease, radiological imaging plays a less prominent role in the percutaneous treatment of valvular and nonvalvular structural disease. Most of these procedures are primarily guided by transesophageal ultrasound, and radiological visualization is the second most-used technique. For this reason, most of these procedures require 2-dimensional transesophageal echocardiography. In some cases, such as with MitraClip implantation or perivalvular leak closure, real-time 3-dimensional transesophageal echocardiography provides certain advantages at different stages of the procedure, which is why this tool is required in centers treating these lesions.22
Another innovative technology now allows the real-time fusion of X-ray fluoroscopy and 2-dimensional images and 2- and 3-dimensional echocardiography images.23,24 Movement of the X-ray tube is synchronized with the transesophageal probe of the echocardiogram, and the different echocardiographic planes are automatically cut according to X-ray tube position. These hybrid techniques make it possible to integrate the 2 image types, which would be helpful at certain steps in procedures such as MitraClip implantation, atrial appendage closure, or perivalvular leak closure.
Requirements of the Cardiac Interventional and Catheterization UnitThe standards document that comprehensively defines the organizational, structural, and resource requirements of cardiac interventional and catheterization units dates from 2001.25 Although many years have passed, many of these recommendations are still valid, and adherence to the guidelines ensures adequate quality of care and safety in clinical and interventional activities.
New recommendations have been published in recent years by both the Spanish Society of Cardiology26 and the American College of Cardiology.27
Life Cycles of Radiology Rooms for Use in Catheterization and Interventional CardiologyAs with other imaging elements, intervention rooms have a life expectancy based on not only age, but also degree of use. Other factors to consider are maintenance, the possibility of part replacement, and the requirements for certain types of treatment.
According to the 2013 guidelines of the Canadian Association of Radiologists,7 the life expectancy of an intervention room is 8 years if the use of the room is high (> 4000 exams/y), 10 if moderate (2000-4000 exams/y), and 12 if low (< 2000 exams/y). However, the life cycle recommendations of other institutions or countries are different; in Spain, the health care unit standards for cardiovascular disease of the Ministry of Health recommend the renovation of catheterization equipment every 7 years.28
Another important aspect is the monitoring and maintenance of equipment to ensure proper functioning and durability and avoid problems arising from equipment failures that may be significant for both patients and health care personnel. Nonetheless, despite the importance of this aspect, according to the Fenin report on the health technology profile in Spain,8 the degree of coverage of the preventive maintenance systems for catheterization laboratories is abnormally low (58%), with all of the implications that this may entail.
Technological Profile in SpainThe technological profile was analyzed according to the methodology described in the previous study by Fenin, entitled “Perfil Tecnológico Hospitalario en España” (Hospital Technological Profile in Spain). In addition, the document on the state of obsolescence of medical imaging technologies in Europe published by COCIR1 has been used, which allows Spain to be placed in the European context.
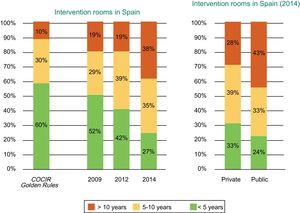
According to the Fenin report,2 the technological profile of the intervention rooms installed in Spain is far from the COCIR recommendations. In 2014, only 27% of the installed equipment was less than 5 years old, 35% between 6 and 10 years, and 38% more than 10 years. In addition, a progressive deterioration in recent years is evident because, in 2009, 52% of the rooms were less than 5 years old, 29% were between 6 and 10 years old, and 19% were more than 10, all of which are much closer to the COCIR guidelines (Figure 8).
Age of the cardiac interventional equipment installed in Spain according to year (2009, 2012, and 2014) and whether the center is public or private. COCIR, European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry. Reproduced with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
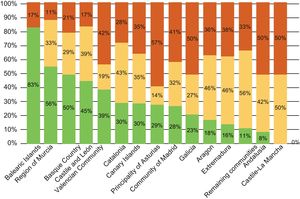
There is also considerable variability within Spain. The autonomous communities of Castile-La Mancha, Andalusia, Extremadura, and Aragon have the highest degree of obsolescence of this type of equipment (Figure 9).
Distribution of the cardiac intervention equipment installed in Spain in 2014 by autonomous community. Reproduced with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
In Europe, the trend has been similar and the age profile of these technologies has also progressively worsened vs previous years. The percentage of equipment more than 6 years old is substantially above 60% in many countries: 77.1% in Greece, 70.7% in Portugal, 69.3% in Denmark, and 66.7% in Spain2 (Figure 10).
Age of the cardiac intervention equipment in Europe in 2014. COCIR, European Coordination Committee of the Radiological, Electromedical, and Healthcare IT Industry. Adapted with permission from the Spanish Federation of Healthcare Technology Companies (Fenin).2
Therefore, evaluation of the potential value and essential elements of each technology would help to ensure quality of care (Table 5).
Equipment Requirements of a Catheterization Laboratory According to its Characteristics
| Characteristics | Standard clinical use | Highly specialized clinical use |
|---|---|---|
| Radiology equipment | Fixed digital angiography equipment | Fixed digital angiography equipment |
| Patient data | Automatic import | Automatic import |
| Coronary quantification | Fractional flow reserve | Fractional flow reserve Intravascular ultrasound or optical coherence tomography |
| Ultrasound | 2-dimensional multiplanar probe | 3-dimensional multiplanar probe |
| Storage | DICOM format PACS | DICOM format PACS |
| Surgery | Transfer to hospital with on-site CVS < 30 min | Cardiopulmonary bypass ≤ 90 min |
CVS, cardiovascular surgery; DICOM, Digital Imaging and Communications in Medicine; PACS, Picture Archiving and Communication System.
The practice of interventional electrophysiology has experienced extraordinary progress during the last 15 years due to the spread of nonfluoroscopic navigation systems and the treatment of complex substrates, such as ventricular tachycardia and atrial fibrillation. The high technical complexity of these procedures is why the material equipment of electrophysiology laboratories is one of the cornerstones of clinical excellence in this field of cardiology.
Physical EquipmentAll electrophysiology units should have the following specifications and components to meet their basic clinical needs:
- •
The physical space must have reasonable access to the intensive care unit, hospital floor, and operating rooms.
- •
There must be 1 or more dedicated intervention rooms, with a surface area of at least 30 m2. If the volume exceeds 200 ablations per year, 2 rooms are recommended.
- •
Electrophysiology laboratories should have the usual resources of an intervention unit, such as standard air conditioning and oxygen, air, and vacuum points. Installation of surgical components is only necessary if the room is used for device implantation.
- •
Given the need for image integration (mainly intracavitary ECG and images from nonfluoroscopic navigation systems and fluoroscopy), it is highly recommended that the room have a single screen showing information from various sources.
- •
A control room, equipped with slave screens for fluoroscopy, recording, and nonfluoroscopic navigation systems, as well as 2 workstations with access to the electronic medical record system of the hospital.
- •
The unit must be equipped with physical space dedicated to patient preparation and/or recovery or the performance of simple techniques, such as electrical cardioversion. This can speed up patient throughput and optimize these highly valued resources.
Although used for both ablation and device implantation in cardiac electrophysiology, fluoroscopy systems play a less prominent role than in the treatment of coronary disease. Most procedures are guided by nonfluoroscopic navigation systems, although fluoroscopy is essential for several stages of the procedure.
- •
All electrophysiology laboratories should be equipped with fixed monoplane digital angiography equipment with basic capabilities. Digital angiography should be considered in centers whose availability of cardiac CT and equipment with biplane technology is subpar, particularly if pediatric procedures are regularly performed.
- •
When there are 2 rooms, with 1 mainly used for device implantation, the second room could be equipped with simpler fluoroscopy equipment, such as a latest-generation portable system (mobile C-arm with image intensifier).
Nonfluoroscopic navigation systems are essential not only to reduce the radiation absorbed by patients and health personnel, but also to ensure the correct 3-dimensional positioning of catheters during intracardiac mapping procedures and the correct localization of the lesions generated.29
In view of its importance, electrophysiology laboratories used for therapeutic ablation procedures must have at least 1 nonfluoroscopic navigation system.
The outfitting of additional systems depends on the volume of the unit and other considerations, such as the existence of research programs, but excessive atomization of procedures through the use of different navigation systems can hinder the accumulation of experience by operators. Installation of an additional nonfluoroscopic navigation system should be considered if a center performs more than 100 ablation procedures per year with nonfluoroscopic navigation.
Additional SystemsIncluded is a series of equipment necessary to perform the most frequent procedures in clinical electrophysiology.
- •
Recording system. The analysis of activation patterns and sequences of intracavitary electrograms remain the basis of the diagnostic and therapeutic approach. Each electrophysiology laboratory should be equipped with a digital recording system with a minimum of 64 intracavitary channels, surface ECG, and a basic hemodynamic module for recording intracavitary pressures. Because intracavitary signals are especially sensitive to noise, the room must be sufficiently shielded to allow the correct recording of multiple signals without interference.
- •
Cryoablation equipment. Cryoablation has advantages over radiofrequency for the management of para-Hisian and atrioventricular nodal reentrant tachycardia pathways in children. It is highly recommended for centers performing more than 200 ablations per year. In atrial fibrillation (AF), it allows procedures with similar outcomes to radiofrequency, but is less demanding for operators. Its incorporation is highly recommended in centers performing more than 50 AF ablations per year. In any case, in centers performing AF ablations, their performance should always be possible by means of a point-to-point ablation procedure guided by a nonfluoroscopic navigation system.
- •
Radiofrequency source. Electrophysiology units must have at least 2 radiofrequency sources with compatible infusion pump systems for irrigated catheters.
- •
Ultrasound console with capacity for transesophageal and intracardiac echocardiography. Basic echocardiography equipment is essential for the early detection of complications, as well as support for both simple techniques, such as transseptal puncture, and more sophisticated techniques, such as intracardiac echocardiography.
- •
Monitor/defibrillator. There must be an external defibrillation system for manual operation in each room.
- •
Robotic navigation systems. Robotic navigation systems are a highly expensive tool of debatable practicality. Its adoption can be considered in high-volume units (more than 250 ablations/y) that have research programs and more than 1 electrophysiology laboratory.
Therefore, evaluation of the potential value and essential elements of each technology would help to ensure quality of care (Table 6).
Minimum Requirements for an Electrophysiology Laboratory
| Standard clinical use | |
|---|---|
| General aspects | HEPA air filters Oxygen, air, and vacuum points Access to hospitalization, ICU, surgery, etc |
| Control room | Slave monitors for fluoroscopy, recording, and nonfluoroscopic navigation systems Workstations with access to the electronic medical record system of the hospital |
| Anteroom space | Patient preparation and/or recovery |
| Radiology equipment | Fixed digital angiography equipment |
| Radiation report | Obligatory |
| Storage | DICOM format PACS |
| Recording system | Digital recording system with at least 64 intracavitary channels and surface ECG Basic intracavitary pressure module |
| Navigation systems | At least 1 nonfluoroscopic navigation system (CARTO, NavX, Rhythmia) |
| Echocardiography | Transthoracic and transesophageal echocardiography Intracardiac echocardiography recommended |
DICOM, Digital Imaging and Communications in Medicine; ECG, electrocardiography; HEPA, high-efficiency particulate air; ICU, intensive care unit; PACS, Picture Archiving and Communication System.
Cardiology as a medical discipline has undergone considerable progress during the last 2 decades. These advances have significantly reduced the mortality rates of the main cardiovascular diseases. This impressive improvement has gone hand-in-hand with technological innovations. The development of new and better equipment has allowed cardiologists to treat more patients and achieve better results. Continuous innovation and appropriate advances in the available technology are essential to further improve clinical practice and guarantee health outcomes.
This report could act as a reference for harmonizing the equipment needed for the main cardiological procedures.
CONFLICTS OF INTERESTNone declared.
.