There is limited evidence regarding the use of subcutaneous implantable cardioverter-defibrillators (S-ICD) in pediatric patients. The aim of this study was to determine the incidence of complications in these patients at our center, according to the type of ICD and patient size.
MethodsWe included all patients aged<18 years who received an S-ICD since 2016 at our center. As a control group, we also included contemporary patients (since 2014) who received a transvenous ICD (TV-ICD). The primary endpoint was a composite of complications and inappropriate shocks.
ResultsA total of 26 patients received an S-ICD (median age, 14 [5-17] years; body mass index [BMI], 20.2 kg/m2). Implantation was intermuscular in 23 patients (88%) and subserratus in the remainder. Two incisions were used in 24 patients (92%). In all patients, 2 zones were programmed: a conditional zone set at 230 (220-230) bpm, and a shock zone set at 250 bpm. Nineteen patients received a TV-ICD (median age, 11 [range, 5-16] years; BMI, 19.2 kg/m2, 79% single-chamber). Survival free from the primary endpoint at 5 years was 80% in the S-ICD group and 63% in the TV-ICD group (P=.54). Survival free from inappropriate shocks was similar (85% vs 89%, P=.86), while survival free from complications was higher in the S-ICD group (96% vs 57%, cloglog P=.016). There were no therapy failures in the S-ICD group, and no increased complication rates were observed in patients with BMI ≤20 kg/m2.
ConclusionsWith contemporary implantation techniques and programming, S-ICD is a safe and effective therapy in pediatric patients. The number of inappropriate shocks is similar to TV-ICD, with fewer short- and mid-term complications.
Keywords
Implantable cardioverter-defibrillators (ICDs) have proven highly effective for preventing sudden death in heart disease patients, and are now the established treatment for this purpose.1–3 ICD therapy is uncommon in pediatric patients because of their low incidence of sudden cardiac death. ICDs are designed for adults, and technical challenges arise when they are used in young patients because of their smaller body size. In addition, associated short- and mid-term complications, mainly related to the electrode, are more frequent in the pediatric population.4 As an alternative to traditional transvenous ICDs (TV-ICDs), subcutaneous ICDs (S-ICDs) implanted in an extrathoracic position attempt to circumvent these complications and avoid possible intravascular infections. Hence, these devices are a particularly attractive option for pediatric patients with a lengthy life expectancy. However, S-ICDs are larger in size, which restricts their use in patients with low body weight, short life expectancy, inability to undergo pacing, and higher rates of inappropriate shocks.5–7 Furthermore, there is still limited experience with S-ICDs in pediatric patients.8–12 Our hypothesis is that S-ICD placement is a safe treatment option for pediatric patients, with a complication rate similar to that of TV-ICDs. The aim of this study is to determine the incidence of ICD-associated complications in patients younger than 18 years in our center, based on the type of device and patient body size.
METHODSStudy designA prospective, ambispective, single-center observational study was conducted in patients younger than 18 years who underwent S-ICD implantation. The comparison group included contemporaneous patients who received a TV-ICD. Follow-up adhered to our center's standard protocol, involving an initial in-person visit after hospital discharge (within the first month postimplantation), followed by remote device monitoring using the Latitude transmission system (Boston Scientific, United States). In the event that transmission failed, patients were contacted by telephone to assess their clinical status and resolve any technical issues. In-person evaluations were scheduled based on the patient's underlying heart condition. TV-ICD patients were prospectively included starting in 2016 and retrospectively enrolled as far as 2014. Two patients who were switched from TV-ICD to S-ICD were included in both groups, taking into account the length of time they belonged to each group. The follow-up protocol was consistent, as described above.
Implantation and programmingThe standard procedure in our center for S-ICD implantation involves generator placement between the latissimus dorsi and serratus muscles using a 2-incision technique, with potential modifications based on the patient's specific characteristics. The electrode was situated in either a left or right parasternal position, depending on the screening results. Fluoroscopy was used before the procedure in all cases to verify correct device positioning and mark the skin. The decision to use fluoroscopy during implantation was left to the operator's discretion. TV-ICD implantation followed the standard technique, leaving a loop in the electrode to accommodate body changes as the patient grew. Antibiotic prophylaxis was used in all procedures.
The typical programming mode for S-ICD included 2 detection zones: shock delivery at 250 beats per minute (bpm) and conditional therapy starting at 220 bpm. The SMART-PASS filter was activated (if available), and postshock pacing was implemented. In TV-ICD, the ventricular fibrillation (VF) window started at 220 bpm, and a monitoring or second therapy zone was chosen at the operator's discretion.
EventsThe primary outcome measure of the study was a composite of complications and inappropriate shocks. As secondary outcomes, we analyzed these events separately, as well as therapy effectiveness, appropriate shocks, and the need and reasons for device replacement. Events were carefully evaluated in the subgroup of patients with a body mass index (BMI) ≤20, as this factor has been associated with an increase in complications in previous studies.12
Complications were categorized into acute events (occurring during the procedure) and events developing after implantation. We analyzed all complications potentially associated with the procedure, the generator, and the electrode. These included pneumothorax, pericardial effusion, tamponade, surgical wound infection, device infection, device dysfunction, need for nonelective device replacement, hematoma requiring a subsequent intervention, electrode displacement requiring a second procedure, therapy failure, and lack of ventricular arrhythmia detection, as well as complications requiring an initially unplanned surgical procedure or device replacement.
Data collectionThe patients’ relevant demographic and clinical data, as well as events occurring during follow-up were recorded. All data were collected using RedCap (Research Electronic Data Capture) forms.13,14
Statistical analysisQualitative data are presented as frequency and percentage, and quantitative data as median [interquartile range]. Variables were compared using the chi-square test and the Wilcoxon rank-sum test, respectively. A survival analysis based on time to event was conducted for time-dependent variables. Kaplan-Meier survival curves were obtained, and differences were analyzed using the log-rank test. To compare survival at a specific time point (eg, at 5 years), we used a test based on cloglog transformation of the Kaplan-Meier estimators.15 Hazard ratios for the primary and secondary events were not calculated because the study variable ICD type violated the proportional hazards assumption of the Cox regression model. Data analysis was performed using R software,16 version 4.2.3, and the following packages: Hmisc (5.0.1), survival (3.5.3), ComparisonSurv (1.1.1), gtsummary (1.7.0) for tables, and ggplot2 (3.4.1) and survminer (0.4.9) for graphs.17–22
Ethical considerationsThe study adhered to the principles of the Helsinki Declaration and was approved by the Research Ethics Committee (CEIm) of the center. Prospectively included patients provided signed informed consent to participate, whereas those included retrospectively were exempted by the CEIm. The image featuring a patient was published after signed consent was obtained from the patient's parents.
RESULTSPatient cohortSince January 2016, S-ICDs have been implanted in 26 patients aged 5 to 17 years, with a minimum weight of 20kg; among them, 12 (46%) had BMI ≤20. In 2 patients, S-ICD implantation was performed because of previous complications with TV-ICDs. Screening results were favorable in at least 2 vectors on 1 side in all except 3 patients, who had only 1 suitable vector on each side. Starting in 2014, TV-ICDs have been implanted in 19 patients (aged 5 to 16 years, minimum weight 24kg; 11 [58%] with BMI ≤20). Since 2016, the reasons for TV-ICD implantation have been a pacing requirement or unfavorable screening for S-ICD placement.
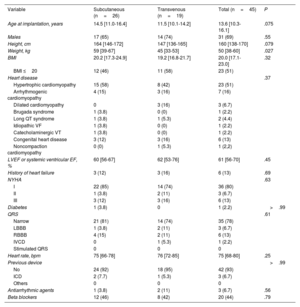
Baseline characteristics of the 2 groups are shown in table 1 and in figure 1 of the supplementary data. Body weight was significantly higher in S-ICD-treated patients then in those receiving a TV-ICD (59kg vs 45kg, respectively; P=.027), although there were no differences in BMI (20.2 vs 19.2; P=.32). In the overall cohort, the predominant underlying heart diseases were hypertrophic cardiomyopathy (51%), arrhythmogenic conditions (16%), congenital heart disease (13%), and ion channel disorders (11%).
Baseline characteristics
| Variable | Subcutaneous (n=26) | Transvenous (n=19) | Total (n=45) | P |
|---|---|---|---|---|
| Age at implantation, years | 14.5 [11.0-16.4] | 11.5 [10.1-14.2] | 13.6 [10.3-16.1] | .075 |
| Males | 17 (65) | 14 (74) | 31 (69) | .55 |
| Height, cm | 164 [146-172] | 147 [136-165] | 160 [138-170] | .079 |
| Weight, kg | 59 [39-67] | 45 [33-53] | 50 [38-60] | .027 |
| BMI | 20.2 [17.3-24.9] | 19.2 [16.8-21.7] | 20.0 [17.1-23.0] | .32 |
| BMI ≤20 | 12 (46) | 11 (58) | 23 (51) | |
| Heart disease | .37 | |||
| Hypertrophic cardiomyopathy | 15 (58) | 8 (42) | 23 (51) | |
| Arrhythmogenic cardiomyopathy | 4 (15) | 3 (16) | 7 (16) | |
| Dilated cardiomyopathy | 0 | 3 (16) | 3 (6.7) | |
| Brugada syndrome | 1 (3.8) | 0 (0) | 1 (2.2) | |
| Long QT syndrome | 1 (3.8) | 1 (5.3) | 2 (4.4) | |
| Idiopathic VF | 1 (3.8) | 0 (0) | 1 (2.2) | |
| Catecholaminergic VT | 1 (3.8) | 0 (0) | 1 (2.2) | |
| Congenital heart disease | 3 (12) | 3 (16) | 6 (13) | |
| Noncompaction cardiomyopathy | 0 (0) | 1 (5.3) | 1 (2,2) | |
| LVEF or systemic ventricular EF, % | 60 [56-67] | 62 [53-76] | 61 [56-70] | .45 |
| History of heart failure | 3 (12) | 3 (16) | 6 (13) | .69 |
| NYHA | .63 | |||
| I | 22 (85) | 14 (74) | 36 (80) | |
| II | 1 (3.8) | 2 (11) | 3 (6.7) | |
| III | 3 (12) | 3 (16) | 6 (13) | |
| Diabetes | 1 (3.8) | 0 | 1 (2.2) | >.99 |
| QRS | .61 | |||
| Narrow | 21 (81) | 14 (74) | 35 (78) | |
| LBBB | 1 (3.8) | 2 (11) | 3 (6.7) | |
| RBBB | 4 (15) | 2 (11) | 6 (13) | |
| IVCD | 0 | 1 (5.3) | 1 (2.2) | |
| Stimulated QRS | 0 | 0 | 0 | |
| Heart rate, bpm | 75 [66-78] | 76 [72-85] | 75 [68-80] | .25 |
| Previous device | >.99 | |||
| No | 24 (92) | 18 (95) | 42 (93) | |
| ICD | 2 (7.7) | 1 (5.3) | 3 (6.7) | |
| Others | 0 | 0 | 0 | |
| Antiarrhythmic agents | 1 (3.8) | 2 (11) | 3 (6.7) | .56 |
| Beta blockers | 12 (46) | 8 (42) | 20 (44) | .79 |
EF, ejection fraction; ICD, implantable cardioverter-defibrillator; IVCD, intraventricular conduction disorder; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional class; RBBB, right bundle branch block; VF ventricular fibrillation; VT, ventricular tachycardia.
Values are expressed as No. (%) or median [interquartile range].
Implantation data and programming at discharge in the 2 groups are described in tables 2 and 3, and in table 1 of the supplementary data. In total, 41 implants (91%) were performed in the electrophysiology room, and 42 (93%) with the patient under general anesthesia. The predominant approach for S-ICD implantation was the 2-incision technique (n=24 [92%]), with intermuscular placement in 23 (88%) patients and subserratus placement in 3 (12%) patients weighing<30kg (figure 1). Fluoroscopy was used during the procedure in 17 patients (65%) to ensure correct electrode positioning. The A219 model was chosen for implantation in all except the first patient in the series, who received the A209 model. Based on the operator's criteria, defibrillation testing was omitted in 1 case due to the patient's clinical condition, and ventricular fibrillation (VF) could not be induced in another patient.
Subcutaneous implantable cardioverter-defibrillator data
| Patients, n | 26 |
| Left screening (suitable vectors) | |
| 1 | 7 (27) |
| 2 | 7 (27) |
| 3 | 12 (46) |
| Right screening (suitable vectors)* | |
| 1 | 3 (14) |
| 2 | 9 (43) |
| 3 | 9 (43) |
| N.oincisions | |
| 2 | 24 (92) |
| 3 | 2 (8) |
| Intermuscular (between serratus and dorsal) | 23 (88) |
| Submuscular (subserratus) | 3 (12) |
| Electrode position | |
| Left parasternal | 22 (85) |
| Right parasternal | 4 (15) |
| Defibrillation test | |
| Not performed | 1 (4) |
| Successful<65 J | 24 (92) |
| VF not inducible | 1 (4) |
| Shock impedance, Ω | 54 [48-68] |
| Therapy time, s | 14.50 [13.35-15.95] |
| Shock window at 250 bpm | 26 (100) |
| Conditional zone | 26 (100) |
| Conditional window, bpm | 230 [220-230] |
| Sensing vector | |
| Primary | 7 (27) |
| Secondary | 12 (46) |
| Alternative | 7 (27) |
| Smart-Pass | 25 (96) |
| Pacing following therapy | 26 (100) |
VF, ventricular fibrillation.
Transvenous implantable cardioverter-defibrillator data
| Patients, n | 19 |
| ICD type | |
| Single chamber | 15 (79) |
| Dual chamber | 4 (21) |
| Resynchronizing | 0 |
| VF window, bpm | 220 [220-226] |
| VT-2 zone | 4 (21) |
| VT-2 window, bpm | 190 [178-200] |
| VT-1 zone | 0 |
| Monitoring zone | 13 (68) |
| Monitoring window, bpm | 180 [170-200] |
ICD, implantable cardioverter-defibrillator; VF, ventricular fibrillation; VT, ventricular tachycardia.
Values are expressed as No. (%) or median [interquartile range].
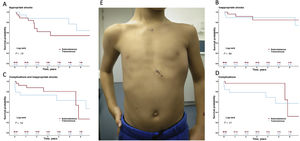
Central illustration. S-ICD implants are safe and effective in children; complication rates and inappropriate shock rates are not higher than with transvenous implantable cardioverter-defibrillators. A-D: Kaplan-Meier curves for appropriate shock-free survival (A), inappropriate shock-free survival (B), complication-free or inappropriate shock-free survival (C), and complication-free survival (D). E: Outcome of subserratus S-ICD implantation in the smallest patient in the series (5 years, 112cm, 20kg). S-ICD, subcutaneous implantable cardioverter defibrillator.
Among TV-ICD-treated patients, 15 (79%) received a single-chamber device. The fluoroscopy time (4.1 [3.0-8.9] vs 0.3 [0.2-0.5] min; P<.001) and procedure duration (90 [71-112] vs 71 [54-75] min; P=.012) were longer in TV-ICD than in S-ICD procedures. No defibrillation tests were carried out.
In all S-ICD patients, 2 zones were programmed, with the shock window threshold set at 250 bpm, and the conditional window at 230 (220-230) bpm. In TV-ICD patients, the VF zone threshold was set at 220 (220-226) bpm, the monitoring zone at 180 (170-200) bpm in 13 (68%) patients, and a second therapy zone was programmed at 190 (178-200) bpm in 4 (21%) patients.
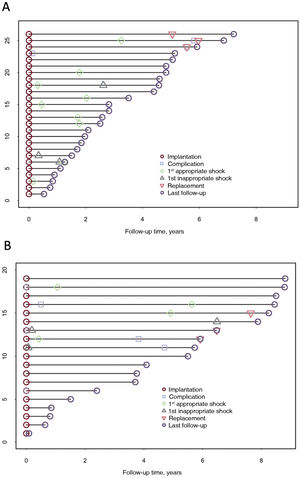
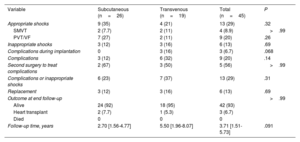
Follow-up and complicationsOverall median follow-up was 3.71 [1.51-5.73] years: 2.70 [1.56-4.77] in the S-ICD group and 5.50 [1.96-8.07] in the TV-ICD group (P=.091). Events are described in table 4, tables 2 to 4 of the supplementary data, figure 1, and figure 2. All patients were alive at completion of the study, and 3 (6.7%) patients had undergone transplantation.
Events and complications during follow-up
| Variable | Subcutaneous (n=26) | Transvenous (n=19) | Total (n=45) | P |
|---|---|---|---|---|
| Appropriate shocks | 9 (35) | 4 (21) | 13 (29) | .32 |
| SMVT | 2 (7.7) | 2 (11) | 4 (8.9) | >.99 |
| PVT/VF | 7 (27) | 2 (11) | 9 (20) | .26 |
| Inappropriate shocks | 3 (12) | 3 (16) | 6 (13) | .69 |
| Complications during implantation | 0 | 3 (16) | 3 (6.7) | .068 |
| Complications | 3 (12) | 6 (32) | 9 (20) | .14 |
| Second surgery to treat complications | 2 (67) | 3 (50) | 5 (56) | >.99 |
| Complications or inappropriate shocks | 6 (23) | 7 (37) | 13 (29) | .31 |
| Replacement | 3 (12) | 3 (16) | 6 (13) | .69 |
| Outcome at end follow-up | >.99 | |||
| Alive | 24 (92) | 18 (95) | 42 (93) | |
| Heart transplant | 2 (7.7) | 1 (5.3) | 3 (6.7) | |
| Died | 0 | 0 | 0 | |
| Follow-up time, years | 2.70 [1.56-4.77] | 5.50 [1.96-8.07] | 3.71 [1.51-5.73] | .091 |
PVT, polymorphic ventricular tachycardia; SMVT, sustained monomorphic ventricular tachycardia; VF, ventricular fibrillation.
Values are expressed as No. (%) or median [interquartile range].
In the S-ICD group, appropriate shock-free survival at 1 and 5 years was 88% and 54%, respectively (table 2 of the supplementary data). Among the 9 patients (35%) who received appropriate shocks, cardioversion was successful at the first attempt (100% effectiveness). In most cases (78%), shocks were delivered to treat polymorphic ventricular tachycardia (VT). None of the patients required switching to TV-ICD for pacing needs, whether for resynchronization, antitachycardia therapy, or bradyarrhythmia. Inappropriate shock-free survival at 1 and 5 years was 96% and 85%, respectively. Three patients (12%) received inappropriate shocks. These were due to T-wave oversensing (n=2), corrected in both patients by optimizing sensing during exercise, and myopotential oversensing (n=1).
Complication-free survival at 1 and 5 years was 96%. There were no procedure-related complications (table 3 of the supplementary data). During the follow-up period, 5 events occurred in 3 patients (12%) (table 4 of the supplementary data): 2 patients experienced surgical wound infections (1 had infection in both wounds) that were resolved with antibiotics and no need for further surgery, and 1 patient required surgical wound cleaning without device removal. In addition, generator replacement was required in 2 patients due to premature battery depletion (both cases were affected by the 92400926D-FA alert). These events occurred after the fifth year of implantation in both patients. The incidence of complications was not higher in patients with BMI ≤20 (figure 2 of the supplementary data).
In the TV-ICD group, 4 patients (21%) experienced appropriate shocks, all of which were successful at the first attempt (100% effectiveness). The underlying cause in 2 patients was sustained monomorphic VT. These patients experienced additional VT episodes that were suppressed by antitachycardia pacing. Seven complications occurred in 6 patients (32%) (complication-free survival rates at 1 and 5 years were 79% and 57%, respectively). Procedure-related complications included 1 pneumothorax and 1 pericardial effusion, managed conservatively, and 1 bleeding episode requiring surgery (table 3 of the supplementary data). During follow-up, 1 patient had undetected VT below the threshold, and 3 patients had out-of-range shock impedance due to electrode stretching caused by growth. Inappropriate therapy was delivered to 3 patients (16%) due to the following events: T-wave oversensing (n=1), sinus tachycardia (n=1), and atrial flutter (n=1) (table 4 of the supplementary data). In the S-ICD group, there were no signal sensing alterations due to growth-related changes in electrode position in the chest (figure 3), even among the 3 patients with a height increase >10cm.
Overall, there were no statistically significant differences between groups in the primary endpoint of the study: event-free survival rates at 1 and 5 years were 92% and 80% in patients receiving S-ICDs, and 73% and 63% in those with TV-ICDs, respectively (figure 1 and table 2 of the supplementary data). At 5 years of follow-up, complication-free survival was higher in the S-ICD group than the TV-ICD group (96% vs 57%; cloglog P=.016), However, later events in the S-ICD group equalized outcomes at the end of follow-up (figure 1D). Differences in complication rates were also observed in the subgroup of patients with BMI<20 (5-year complication-free survival 100% vs 48%; P=.037) (figure 3). Inappropriate shock rates were similar in the 2 groups (figure 1 and table 2 of the supplementary data).
DISCUSSIONThis study compares the outcome of S-ICD implantation (2-incision technique with intermuscular or subserratus positioning and therapy zones programmed at high frequencies), with the results of traditional TV-ICD implantation, exclusively in pediatric patients (age < 18 years). In addition, it marks the first experience in Spain of S-ICD use in this age group. The main results are as follows: a) S-ICD implantation was effective for treating ventricular arrhythmia; b) there were no implantation-related complications or electrode dysfunction; c) the inappropriate shock rate associated with S-ICDs was similar to that of TV-ICDs; d) patients with BMI ≤20 did not have higher complication rates, and e), there were fewer complications with S-ICD than with TV-ICD implants at short- and mid-term. Nonetheless, after the fifth year of follow-up, generator replacement was required in 2 S-ICD patients due to premature battery depletion. Therefore, despite a lower incidence of events compared with the TV-ICD group, the differences did not attain statistical significance in the survival curves. Taken together, these results suggest that S-ICDs could be considered the device of choice for pediatric patients, provided they are suitable candidates for the implant.
TV-ICD implantation is an effective, widely recognized therapy for the prevention of sudden cardiac death. These devices are, by far, the most commonly used in our setting,23 but they are not without risks or significant complications, which should be taken into consideration. Complications can occur during the procedure (eg, pneumothorax, perforation), as well as at long-term, mainly in relation to the intravascular nature of the device and its most fragile component, the electrode. In extended follow-up periods, electrode dysfunction is a common event (15%-40%),24,25 particularly in pediatric patients due to their growth during development.4 Thus, it is an expected complication in these patients, who, additionally, have a long life expectancy. Another serious complication is device infection, which has a significant impact on morbidity, mortality, and cost related to hospitalization and additional procedures.26–29 Of note, epicardial devices are not an optimal alternative, as they are associated with an even higher complication rate than TV-ICDs.30
To address these drawbacks, S-ICDs have emerged, devices allowing complete extravascular placement. Previous reports have shown that S-ICDs are safe and effective,5 with fewer associated electrode-related complications than TV-ICDs, albeit with higher rates of inappropriate shocks.6 Only 1 related randomized clinical trial has been conducted to date, which found that S-ICDs were not inferior to TV-ICDs in patients not requiring pacing.7 However, the published experience in pediatric patients remains limited.8–12
The effectiveness of S-ICD implants was substantiated in some of the first pediatric series. Nonetheless, device-related complications associated with the initial surgical technique (3 incisions, shallower implantation) were also reported, particularly in patients with low BMI.8–12 Two multicenter studies were recently published in this line,10,12 although they show some differences with respect to the present series (table 5 of the supplementary data). The first study, conducted in centers across the United States, Canada, and China, included 115 patients. They were not limited to pediatric cases, but also included adults with congenital heart conditions (median age, 17 years; weight, 71kg). The 2-incision technique was used in 47%, and intermuscular implantation was performed in only 18%.10 The second was the SIDECAR study, conducted in various European centers, which included 81 patients aged 8 to 17 years.12 In both studies, the cumulative incidence of surgical complications was 4% to 11%, and inappropriate shocks occurred in 19% to 21% of patients after a 3-year follow-up period. These complications were generally more common in patients with low BMI (<20).
The complication rate was lower in the present study than in previous reports on S-ICD use, even though the patients included were younger and had lower body weight (table 5 of the supplementary data). The reasons for this difference are likely multifactorial. For example, the implant technique used, except in a few cases, involved 2 incisions and deep placement of the device (intermuscular or even subserratus in smaller patients). In addition, adoption of S-ICD implantation in Spain occurred later than in other countries in our region. This allowed us to benefit from the experience of other groups and incorporate ongoing improvements in the technique right from the beginning. Lastly, there were no complications related to growth during development, even in patients who experienced significant height increases.
The inappropriate shock rate associated with S-ICDs was comparable to the rate for TV-ICDs in our study. Programming was carried out using high detection windows (VF zone>250 bpm and conditional zone ≥220 bpm) and the SMART-PASS filter to minimize these events. However, in patients with TV-ICDs, threshold frequencies for the VF zone (median, 220 bpm), and the VT zone (median, 190 bpm) in those with this feature activated, were not as high as in patients with SC-ICDs. This difference could have had an impact on the incidence of inappropriate shocks in this subgroup.
As was seen in this study, one of the main advantages of SC-ICDs over TV-ICDs is that infections can be treated, at least initially, with antibiotics. Hence, there is often no need for device extraction, a procedure associated with considerable morbidity for the patient and cost for the health care system. Again, there were no issues related to the electrode despite growth, in contrast to what occurred in some patients with TV-ICDs. Finally, improvements are expected in SC-ICD technology, such as smaller device size and longer battery life, the main limitations affecting pediatric patients. These advances may contribute to reducing long-term events.
LimitationsSome limitations of this study should be acknowledged. First, the research was based on a single-center registry from a tertiary center with experience in pediatric device implantation, and procedures were carried out by a small number of operators. These factors may restrict general applicability of the results. Second, the small sample size and existing selection bias (regarding type of device) greatly limit conclusions drawn from the statistical comparisons. Hence, it would be advisable to conduct multicenter studies in this line. In addition, it could be worthwhile to consider including devices implanted in pediatric patients in the National ICD Registry23 published by the Arrhythmia Association of the Spanish Society of Cardiology, to collect data from other centers in Spain. Third, the follow-up time, although longer than in other studies, may have been insufficient to detect some long-term complications, especially concerning device battery life and potential electrode-related events. Lastly, factors that could have an impact on deciding the choice of device, such as quality of life, and others, such as cost-effectiveness, were not assessed in this study.
CONCLUSIONSS-ICD implantation using a 2-incision procedure with intermuscular positioning (subserratus in patients with very low body weight) and programming with high-frequency sensing windows offers a favorable safety and effectiveness profile for preventing sudden cardiac death in pediatric patients. This approach circumvents potential complications associated with the intravascular electrodes used in traditional TV-ICDs. S-ICD implantation could be the treatment of choice in children and adolescents, even those with BMI<20, although further experience is needed to confirm the results at mid- and long-term.
- –
ICD implantation in pediatric patients is a complex procedure with higher complication rates than in adults.
- –
The pediatric population is exposed for a much longer time to potential long-term complications, mainly those associated with intravascular electrodes.
- –
Previous studies including young patients have shown that S-ICDs are safe and effective in this population, although complication rates are higher than with TV-ICDs, especially in patients with BMI ≤20.
- –
There are no previous studies comparing outcomes between S-ICDs and TV-ICDs exclusively in pediatric patients.
- –
S-ICDs with intermuscular or subserratus implantation, preferably using a 2-incision technique and programming 2 high-frequency zones, is a good alternative to TV-ICD use in pediatric patients. S-ICDs were associated with fewer short- and mid-term complications and showed a similar inappropriate shock rate.
- –
There were no complications related to patient growth during follow-up.
- –
In contrast to the results of previous studies, the complication rate was not higher in patients with BMI ≤20 vs BMI>20 in patients receiving S-ICDs with current implantation techniques. Furthermore, there were significantly fewer complications overall than with TV-ICDs.
This study was carried out at the initiative of the researchers and received no financial support.
AUTHORS’ CONTRIBUTIONSStudy conception and design: M. Centeno, R. Álvarez García-Rovés and P. Ávila. Patient enrollment and data collection: all authors. Project supervision and revision of the original: A. Arenal, F. Fernández-Avilés, and C. Medrano. Statistical analysis: P. Ávila. Preparation of the first draft and important contributions: all authors. All authors have agreed to the content of the final version of the manuscript. M. Centeno and R. Álvarez are the first coauthors.
CONFLICTS OF INTERESTP. Ávila has received fees for consultancy and teaching from Boston Scientific and Metronic. Á. Arenal has received consultancy fees from Boston Scientific and Metronic. F. Atienza and A. Carta have received consultancy fees from Medtronic. The remaining authors declare no conflicts of interest.