Sudden cardiac death is the most common medical cause of death during the practice of sports. Several structural and electrical cardiac conditions are associated with sudden cardiac death in athletes, most of them showing abnormal findings on resting electrocardiogram (ECG). However, because of the similarity between some ECG findings associated with physiological adaptations to exercise training and those of certain cardiac conditions, ECG interpretation in athletes is often challenging. Other factors related to ECG findings are race, age, sex, sports discipline, training intensity, and athletic background. Specific training and experience in ECG interpretation in athletes are therefore necessary. Since 2005, when the first recommendations of the European Society of Cardiology were published, growing scientific evidence has increased the specificity of ECG standards, thus lowering the false-positive rate while maintaining sensitivity. New international consensus guidelines have recently been published on ECG interpretation in athletes, which are the result of consensus among a group of experts in cardiology and sports medicine who gathered for the first time in February 2015 in Seattle, in the United States. The document is an important milestone because, in addition to updating the standards for ECG interpretation, it includes recommendations on appropriate assessment of athletes with abnormal ECG findings. The present article reports and discusses the most novel and relevant aspects of the new standards. Nevertheless, a complete reading of the original consensus document is highly recommended.
Keywords
Interpretation of electrocardiogram (ECG) findings is an essential skill for any physician involved in the cardiac evaluation of athletes. The European Society of Cardiology Society guidelines recommend ECG testing in all screening programs performed prior to the practice of sport.1 The use of ECG as a basic screening test for athletes, alongside clinical history and physical examination, is justified by its ability to detect cardiomyopathies and channelopathies, which are the main causes of sudden cardiac death (SCD) in athletes younger than 35 years. The cost-effectiveness of this test has also been demonstrated in studies of European athletes.2 Electrocardiogram interpretation, however, is not easy, as manifestations of athlete's heart, ie, physiological adaptations to an athlete's heart as a result of exercise, can resemble changes seen in cardiomyopathies and channelopathies.
Numerous revisions aimed at improving the specificity of ECG interpretation, while maintaining its high sensitivity, for the detection of cardiopathies associated with SCD,3–8 have appeared since the European Society of Cardiology published its first recommendations for ECG interpretation in 2005.1 Some of the revised recommendations were included in consensus documents published following the Summit on ECG Interpretation in Athletes, which brought together experts in sports cardiology and medicine in Seattle in 2012.4–7 The Seattle Criteria were the most widely used recommendations on ECG interpretation in athletes until the recent publication of the new international consensus statement that emerged from the 2015 consensus summit, also held in Seattle.9–11 This new document has 3 main objectives: a) to update ECG interpretation standards based on new research and up-to-date evidence, b) to develop a clear guide to the appropriate evaluation of ECG abnormalities for conditions associated with SCD in athletes, and c) to help physicians take clinical decisions based on the characteristics of each athlete.
It should be clarified that the document is aimed not only at clinical cardiologists, but also at a wide range of specialists involved in the medical care of athletes, such as general practitioners, pediatricians, and sports physicians. In brief, the additional clinical evaluation and management recommendations are designed to help a wide range of health care professionals to take better clinical decisions related to the health of the athletes under their care. Because many of the recommendations are based on consensus opinions reached at the 2015 summit, the authors stress that the document is designed to serve as a guide and that physicians should also base their decisions on their own experience and the individual characteristics of each athlete. The systematic use of these new criteria can be expected to further improve their reliability and reduce interobserver variability.
In this article, we discuss the key features of the new consensus document, highlighting both changes to previous criteria and more challenging aspects of ECG interpretation.
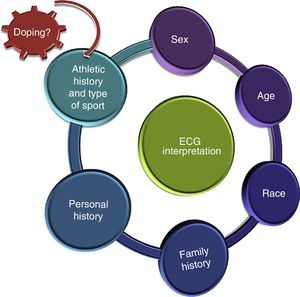
The Importance of Who and HowThe document underlines the importance of interpreting ECG findings within the context of each athlete. In other words, the ECG should not be used as an isolated diagnostic test. Factors that can influence the prevalence of certain ECG abnormalities in athletes are age, sex, race, type and intensity of training, and athletic history (Figure 1). Electrocardiogram abnormalities in athletes who have not been training for long or exposed to intense training should be interpreted carefully and not immediately attributed to physiological alterations induced by exercise.
One of the novel aspects of the new consensus standards is the inclusion of recommendations for athletes aged 12 to 16 years (juvenile pattern) and those aged 30 years or older. This second group has a considerably increased prevalence of heart disease.
The decision to order additional tests is determined by personal history (appearance of symptoms such as syncope or presyncope, chest pain, dyspnea, or palpitations largely during exercise), a family history of hereditary cardiovascular disease or sudden death, and/or an abnormal physical examination, even when accompanied by normal ECG findings. The authors also recall that some of the diseases associated with SCD in athletes, such as congenital anomalous coronary arteries, premature coronary atherosclerosis, and aortopathies, are rarely accompanied by abnormalities in the baseline ECG.
Another noteworthy feature of the document is the importance it gives to proper ECG preparation and lead placement, as incorrect positioning can affect interpretation and yield false-positive results, such as pseudo-Q waves, failure to detect ST-segment depression (due to downward misplacement of precordial leads), pseudo–ST-segment elevation simulating myocardial injury, pericarditis or the Brugada type 2 pattern (due to upward misplacement of precordial electrodes), negative P waves, a negative QRS complex, and T-wave inversion (TWI) in leads I and aVL but not in V5-V6 (due to reversal of the right and left arm leads).
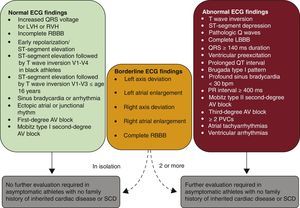
Changes to ECG Interpretation Criteria in Athletes: Normal and Borderline FindingsThe document classifies the main ECG findings in athletes according to whether they are considered normal, borderline, or abnormal (Figure 2). The addition of the borderline category is one of the main modifications to the document. This new category includes a subgroup of ECG alterations that were previously classified as abnormal, but may now be interpreted as normal or abnormal depending on whether or not they are accompanied by other findings. Although this is an important change with respect to the original Seattle criteria,4–7 this borderline category had already been described and validated.8,12
International consensus standards for ECG interpretation in athletes. AV, atrioventricular; ECG, electrocardiogram; LBBB, left bundle branch block; LVH, left ventricular hypertrophy; PVC, premature ventricular contraction; RBBB, right bundle branch block; RVH, right ventricular hypertrophy; SCD, sudden cardiac death. Adapted from Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: Consensus statement. Br J Sports Med. 2017;51:704–731 with permission from BMJ Publishing Group Ltd.9.
Normal ECG findings comprise a wide range of electrical manifestations associated with athlete's heart that do not require additional diagnostic testing (Figure 2 and Table 1). The following findings previously classified as abnormal are now considered to be normal in the new document: a) isolated QRS voltage criteria for right ventricle (RV) hypertrophy (RV1 + SV5 or SV6 >1.1mV), bringing it on a par with isolated QRS voltage criteria for left ventricle hypertrophy (SV1 + RV5 or RV6 >3.5mV), in the absence of other clinical markers or ECG findings that indicate disease (inferolateral TWI, ST-segment depression, and pathological Q waves) and b) TWI or biphasic T wave in V1 to V3 in adolescents younger than 16 years or in prepubertal adolescents (juvenile pattern).
Definition of Normal and Borderline Findings for ECG Interpretation in Athletes According to the 2017 International Consensus Standards
| Borderline ECG findings in athletes | |
|---|---|
| These ECG findings in isolation likely do not represent pathological cardiovascular disease in athletes, but the presence of 2 or more borderline findings calls for additional investigation | |
| ECG abnormality | Definition |
| Left axis deviation | −30° to −90° |
| Left atrial enlargement | Prolonged P wave duration of >120 ms in leads I or II with negative portion of the P wave ≥1mm in depth and ≥40ms in duration in lead V1 |
| Right axis deviation | > 120° |
| Right atrial enlargement | P wave ≥2.5mm in II,III, or aVF |
| Complete right bundle branch block | rSR’ pattern in lead V1 and S wave > R wave in lead V6 with QRS duration ≥120m |
| Normal ECG findings in athletes | |
|---|---|
| These ECG alterations are physiological adaptations to regular exercise; they are considered normal variants in athletes and do not require further evaluation in asymptomatic athletes with no significant family history | |
| Normal ECG finding | Definition |
| Increased QRS voltage | Isolated QRS voltage criteria for left (SV1 + RV5 or RV6 >3.5mV) or right ventricular hypertrophy (RV1 + SV5 or SV6 >1.1mV) |
| Incomplete right bundle branch block | rSR’ pattern in lead V1 and a qRS pattern in lead V6 with QRS duration <120ms |
| Early repolarization | J-point elevation, ST-segment elevation, J waves, or terminal QRS slurring in the inferior and/or lateral leads |
| Black athlete repolarization variant | J-point elevation and convex (domed) ST-segment elevation followed by T-wave inversion in leads V1-V4 in black athletes |
| Juvenile T-wave pattern | T-wave inversion V1-V3 in athletes aged < 16 years |
| Sinus bradycardia | ≥30bpm |
| Sinus arrhythmia | Heart rate variation with respiration: rate increases during inspiration and decreases during expiration |
| Ectopic atrial rhythm | P waves are a different morphology compared with the sinus P wave, such as negative P waves in the inferior leads (low atrial rhythm) |
| Junctional escape rhythm | QRS rate is faster than resting P wave or sinus rate and typically less than 100bpm with narrow QRS complex unless the baseline QRS is conducted with aberrancy |
| First-degree atrioventricular block | PR interval 200-400ms |
| Mobitz type I (Wenckebach) second-degree atrioventricular block | PR interval progressively lengthens until there is a nonconducted P wave with no QRS complex; the first PR interval after the dropped beat is shorter than the last conducted PR interval |
ECG, electrocardiogram.
Adapted from Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: Consensus statement. Br J Sports Med. 2017;51:704-731 with permission from BMJ Publishing Group Ltd.9.
The following normal ECG findings remain unchanged: a) sinus bradycardia, lengthening of PR interval, and situations such as Mobitz type I second-degree atrioventricular block, ectopic atrial rhythm, and junctional escape (nodal) rhythm, as long as they normalize with exercise; b) incomplete right bundle branch block; c) early repolarization patterns in the absence of other ECG abnormalities or clinical markers of disease; and d) TWI in V1 to V4 preceded by J-point elevation and convex ST-segment elevation, again in the absence of other ECG or clinical findings.
Borderline FindingsThe new borderline category includes findings that were previously considered abnormal (Figure 2 and Table 1). In the new system, borderline findings are considered to be normal when they occur in isolation. They are attributed to physiological remodeling and do not require further testing. Detection of 2 or more borderline findings could indicate underlying disease and always calls for further investigation.
Two aspects in this category are worth commenting on briefly. First, according to recent studies, axis deviation and voltage criteria for atrial enlargement are not correlated with structural heart disease. In 1 study, consideration of these findings as normal when they occurred in isolation reduced the false-positive rate from 13% to 7.5% and improved specificity from 90% to 94%, while only minimally reducing sensitivity (91%-89.5%).13 The second aspect is related to complete right bundle branch block. While incomplete block is common in athletes, the significance of complete block is more uncertain. In a recent study of 510 athletes, complete right bundle branch block (present in 2.5% of athletes) was associated with larger RV dimensions and a lower right ejection fraction but not with structural heart disease. Complete right bundle branch block is thus considered to be a manifestation of physiological remodeling (RV dilation, QRS prolongation, and a relative reduction in RV systolic function at rest).14
Abnormal ECG Findings: When Is an ECG Insufficient?With the exception of several criteria that were switched to the borderline category, few changes were made to the abnormal ECG category in the new document.4–6,8 One major change, however, is that the authors do not just describe the abnormalities (Figure 2 and Table 2) but also provide guidelines for specific diagnostic and clinical actions to follow on their detection. This is one of the strengths of the document.
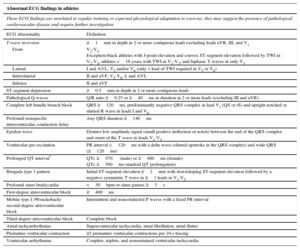
Definition of Abnormal Findings for ECG Interpretation in Athletes According to the 2017 International Conensus Standards
| Abnormal ECG findings in athletes | |
|---|---|
| These ECG findings are unrelated to regular training or expected physiological adaptation to exercise; they may suggest the presence of pathological cardiovascular disease and require further investigation | |
| ECG abnormality | Definition |
| T-wave inversion | ≥1mm in depth in 2 or more contiguous leads excluding leads aVR, III, and V1 |
| Front | V2-V4 Exception:black athletes with J-point elevation and convex ST-segment elevation followed by TWI in V2-V4; athletes <16 years with TWI in V1-V3; and biphasic T waves in only V3 |
| Lateral | I and AVL, V5 and/or V6 (only 1 lead of TWI required in V5 or V6) |
| Inferolateral | II and aVF, V5-V6, I, and AVL |
| Inferior | II and aVF |
| ST-segment depression | ≥0.5mm in depth in 2 or more contiguous leads |
| Pathological Q waves | Q/R ratio ≥0.25 or ≥40ms in duration in 2 or more leads (excluding III and aVR) |
| Complete left bundle branch block | QRS ≥120ms, predominantly negative QRS complex in lead V1 (QS or rS) and upright notched or slurred R wave in leads I and V6 |
| Profound nonspecific intraventricular conduction delay | Any QRS duration ≥140ms |
| Epsilon wave | Distinct low amplitude signal (small positive deflection or notch) between the end of the QRS complex and onset of the T wave in leads V1-V3 |
| Ventricular pre-excitation | PR interval <120ms with a delta wave (slurred upstroke in the QRS complex) and wide QRS (≥120ms) |
| Prolonged QT interval* | QTc ≥470 (male) or ≥480ms (female) QTc ≥500ms (marked QT prolongation) |
| Brugada type 1 pattern | Initial ST-segment elevation ≥2mm with downsloping ST-segment elevation followed by a negative symmetric T wave in ≥1 leads in V1-V3 |
| Profound sinus bradycardia | <30bpm or sinus pauses ≥3s |
| First-degree atrioventricular block | ≥400ms |
| Mobitz type I (Wenckebach) second-degree atrioventricular block | Intermittent and nonconducted P waves with a fixed PR interval |
| Third-degree atrioventricular block | Complete block |
| Atrial tachyarrhythmias | Supraventricular tachycardia, atrial fibrillation, atrial flutter |
| Premature ventricular contraction | ≥2 premature ventricular contractions per 10-s tracing |
| Ventricular arrhythmias | Couplets, triplets, and nonsustained ventricular tachycardia |
ECG, electrocardiograma, QTc, computer-derived QT.
The QT interval corrected for heart rate is ideally measured using Bazett's formula with heart rates between 60 and 90 bpm, preferably performed manually in lead II or V5 using the teach-the-tangent method15 to avoid inclusion of a U wave. Consider repeating the ECG after mild aerobic activity for a heart rate <50 bpm or after a longer resting period for a heart rate > 100bpm if the QTc value is borderline or abnormal.
Adapted from Drezner JA, Sharma S, Baggish A, et al. International criteria for electrocardiographic interpretation in athletes: Consensus statement. Br J Sports Med. 2017;51:704-731 with permission from BMJ Publishing Group Ltd.9
In this next section, we discuss aspects that are of particular interest because they were modified or because they entail greater interpretation challenges. For a more detailed review of the scientific evidence supporting the recommendations, we advise consulting the original document.9–11 Generally speaking, abnormal ECG findings in athletes can be classified into 3 subgroups depending on the suspected underlying diseases: structural disease, primary arrhythmia or channelopathy, and rhythm disorders and ventricular pre-excitation.
Alterations Suggestive of Structural DiseaseT wave inversion is one of the most clinically relevant ECG findings and is one of the most difficult to interpret. Its pathological significance is determined by location, age, sex, and race.
Lateral TWI (I and aVL, V5 and/or V6—just 1 deviation is necessary) and inferolateral TWI are always considered abnormal and indicate underlying cardiomyopathy16–19 or myocarditis. Athletes with lateral or inferolateral TWI need to be thoroughly investigated by cardiac magnetic resonance (CMR), particularly in the presence of deep TWI or significantly decreased ST-segment depression. Additional tests include echocardiography, Holter monitoring, and an exercise stress test.20 A family study is recommended and genetic testing should also be contemplated.21 If a definitive diagnosis is not established, the athlete should be referred for annual follow-up to investigate phenotypic expressions of cardiomyopathy.18,19 Anterior TWI (V1-V4) is more difficult to evaluate and identification of the underlying pathology can be aided by consideration of factors such as race, age, sex, and preceding ST-segment characteristics. According to a recent study, anterior TWI preceded by J-point elevation of 1mm or more rules out a diagnosis of cardiomyopathy with a negative predictive value of 100% in athletes of any race,22 and particularly in athletes who practice endurance sports.23 TWI confined to V1-V2 may also be a normal finding and is more common in female athletes (prevalence of 1% vs 0.2% for male athletes).24 Additional studies are needed to rule out arrhythmogenic RV cardiomyopathy (ARVC) in most nonblack athletes aged 16 or older with TWI beyond V2. If this finding is accompanied by J-point elevation, ST-segment elevation, or biphasic T waves, it is more likely to be the expression of physiological changes induced by exercise. However, if it occurs in the absence of J-point elevation (<1 mm) or in the presence of ST-segment depression, it should be considered abnormal and ARVC must be ruled out. The extent of subsequent investigations (echocardiography, CMR, Holter monitoring, exercise stress testing, and signal-averaged ECG) will be determined by the presence of a relevant family history and other ARVC-related ECG findings (epsilon waves, low voltage in limb leads, prolonged S wave upstroke, and premature ventricular contractions [PVCs] with left bundle branch block).25 Although the significance of TWI in the inferior leads (II, III, or aVF) is unknown, this finding should not be attributed to physiological remodeling and warrants, at least, further evaluation by echocardiography and annual follow-up.
ST-segment depression (relative to the isoelectric PR segment) over 0.05mV (0.5mm) in 2 or more leads is a common finding in hypertrophic cardiomyopathy (HCM).26,27 Patients with this finding should be referred for at least an echocardiogram and depending on the results and the accompanying clinical findings, CMR.
Pathological Q waves may be detected in patients with cardiomyopathy (HCM, dilated cardiomyopathy, noncompaction), myocarditis, or a history of acute myocardial infarction. They may also be seen in accessory pathways (look for delta waves and evaluate the PR interval) or in cases of lead misplacement. Correct lead positioning should be checked on detection of a QS pattern in V1-V2. In an attempt to improve the specificity of ECG, pathological Q waves are now defined by a Q:R ratio of 0.25 or higher rather than a Q-wave voltage of 3mm or deeper.4 Q waves lasting at least 40ms are still considered to be pathological and in both cases the waves must be detected in 2 or more contiguous leads (excluding III and aVR.) Echocardiography, a thorough family history, and evaluation of cardiovascular risk factors should be performed in all athletes with pathological Q waves, particularly when they are aged 30 years or older. An exercise stress test is warranted on detection of multiple cardiovascular risk factors or suspicion of previous acute myocardial infarction. Cardiac magnetic resonance, in turn, is indicated in athletes with pathological Q waves accompanied by ST-segment depression, TWI, or suspicious clinical findings.
Structural heart disease must be ruled out on detection of complete left bundle branch block,28 profound nonspecific intraventricular conduction delay (QRS ≥140ms), or epsilon waves, as these are major criteria for ARVC.25
In all cases, a full cardiac evaluation with imaging studies is recommended to rule out structural heart disease. Echocardiography is always recommended, and CMR is particularly advisable in cases of suspected ARVC and apical HCM, which can be more difficult to detect by echocardiography.29 The extent of the study will be determined by the presence of symptoms and/or a relevant family history. In patients with a family history, genetic testing can help to identify individual risk in certain types of heart disease.
Alterations Suggestive of a ChannelopathyNo changes were made to the threshold for a normal QT interval in the new document (470ms for male athletes and 480ms for female athletes). The document, however, underlines the importance of accurate measurement and manual confirmation of computer-derived QT interval (QTc). In patients with a prolonged QTc, reversible causes should be ruled out. These include electrolyte abnormalities (hypokalemia, hypomagnesemia) and use of drugs that can lengthen the interval. Additional investigation is not necessary in patients with a negative personal and family history and a normal QTc on a repeat ECG. If the QTc is still elevated on the second ECG, ECG testing of all first-degree relatives is recommended and the athlete should be referred to a heart rhythm specialist. Athletes with a QTc of 500ms or higher and no identifiable reversible causes should be seen by a specialist.30–32
Given the extremely low prevalence of short QT intervals (<320ms) and the absence of data indicating morbidity in asymptomatic athletes,33 the authors of the document recommend the performance of additional tests only if the finding is accompanied by syncope, premature atrial fibrillation, ventricular arrhythmias, or a relevant family history.
The document focuses on type 1 Brugada pattern (coved rSr’ pattern, ST-segment elevation of 2mm or higher, and inversion of the terminal portion of the T wave in V1, V2, and V3). Correct placement of V1 and V2 leads (in the fourth intercostal space) must be checked, as placement in the second and third spaces may not only accentuate type 1 pattern but also produce patterns similar to type 2 Brugada patterns34. The document recommends using the Corrado index (ST-segment elevation at J-point [STJ]/ST-segment elevation at 80ms [ST80])35 to differentiate between early repolarization of athlete's heart and the type 1 Brugada pattern (STJ/ST80 >1). Although black athletes and endurance athletes may present repolarization changes similar to those seen in type 1 Brugada pattern, additional studies are only recommended in athletes with symptoms or a relevant history.
Imaging studies do not have diagnostic value in the above cases, and Holter monitoring and stress tests are essential for detecting ventricular arrhythmias and evaluating QT behavior. A drug challenge test and an electrophysiological study may be necessary, and, if there is a positive family history, genetic testing can help to determine individual risk.21
Rhythm Disorders and Ventricular Pre-excitationThe new consensus document continues to recommend assessment of chronotropic response to aerobic activity in athletes with a resting heart rate of 30bpm or sinus pauses of 3seconds or longer. If the response is inadequate or if the athlete reports presyncope or syncope, primary sinus node disease must be ruled out. A PR interval or 400ms or longer requires additional evaluation, although exposure to mild aerobic exercise may be sufficient to check for shortening of the interval. Both Mobitz type II second-degree and third-degree (complete) AV block are still considered abnormal findings.
Detection of 2 or more PVCs per 10-second tracing on a baseline ECG continues to be considered abnormal and warrants further investigation via ambulatory ECG monitoring, echocardiography, and an exercise stress test. Additional testing is not necessary if the results of the ambulatory monitoring and echocardiography are normal and if the PVCs are suppressed by exercise and there are no symptoms. However, contrast-enhanced CMR and electrophysiological evaluation are recommended on detection of 2000 or more PVCs in 24hours, nonsustained ventricular tachycardia, or an increase in PVCs during an incremental exercise test.36 The authors of the document are of the opinion that RV disease should be investigated in high-dynamic athletes with a single PVC showing left bundle branch block morphology and superior axis, especially when they are aged 25 years or older.
Supraventricular tachycardias, atrial fibrillation, and atrial flutter are rare on baseline ECG in young athletes37 and must be investigated. Although these arrhythmias are generally benign, athletes tend to have symptoms that are occasionally associated with diseases that can cause SCD (myocarditis, Brugada syndrome, long QT syndrome, Wolff-Parkinson-White syndrome, congenital diseases, and cardiomyopathies).
The risk of accessory pathway should be evaluated, generally via an exercise stress test, in athletes with asymptomatic ventricular pre-excitation. If the results are inconclusive or if the athlete is a competitive athlete who practices a moderate-intensity or high-intensity sport, electrophysiological evaluation is warranted. An echocardiogram should also be contemplated given the association between Wolff-Parkinson-White syndrome and Epstein anomaly and cardiomyopathy.
Special Considerations and Recommendations for Sports PracticeThe International Criteria for Electrocardiographic Interpretation in Athletes statement is the first document of its type to include specific recommendations for ECG interpretation in athletes aged 30 years or older and in adolescents younger than 16 (which we have already discussed). Considering that the main cause of SCD in people over 30 years is ischemic cardiomyopathy, the document recommends considering changes in baseline ECG that could indicate underlying heart disease (pathological Q waves, TWI, ST-segment depression, bundle branch blocks, abnormal R wave progression, anterior hemiblock, and atrial fibrillation).38 In such cases it is important to investigate cardiovascular risk factors and to determine the need for functional or imaging studies on a case-by-case basis.
Recommendations for sports participation are always important, and even more so in equivocal or borderline cases due to the potential medical, legal, or psychological ramifications. Temporary restriction from activity in athletes with significant ECG alterations should be considered. Careful scheduling of preparticipation screening should help to ensure relatively rapid testing and minimize the impact of this restriction. As a general rule, cessation of activity and subsequent re-evaluation in athletes with abnormal ECG findings is not recommended, as it is a difficult measure to implement in competition sports.
Certain ECG abnormalities can precede the structural development of HCM, ARVC, or familial dilated cardiomyopathy in genetically predisposed athletes; approximately 6% of athletes with abnormal ECG findings were found to develop features of these cardiomyopathies during follow-up.18,19 All asymptomatic athletes with an inconclusive diagnosis and ECG abnormalities that raise suspicion of structural cardiomyopathy should be scheduled for annual follow-up (including imaging studies) both during and after their sporting careers. They should be alerted to the importance of lifetime follow-up and encouraged to report any new symptoms. Symptomatic athletes, ie, those who experience chest pain, dyspnea, palpitations, syncope, or seizures during exercise, should be evaluated, even if they have normal ECG findings. Genetic testing in asymptomatic athletes with a family history of SCD or a heritable cardiovascular disease can help to determine individual risk of cardiovascular disorders, such as HCM, ARVC, and long QT syndrome.
Finally, the new consensus statement highlights the importance of a multidisciplinary approach to the care of athletes with heart disease and those advised to withdraw from competition due to a cardiovascular abnormality. In such cases, particular attention should paid to psychological evaluation and support, which should include adequate professional and/or athletic reorientation due to the increased risk of psychological distress.39 Athletes advised to give up competitive sport should be counseled on the most suitable type, frequency, and duration of physical activity or sport according to their underlying type of heart disease.
Future Prospects and ConclusionsThe revised international consensus document is an important educational tool targeting all types of physicians involved in the care of athletes. It will advance knowledge of the evaluation and interpretation of ECG abnormalities and form a cornerstone for improving the quality of cardiovascular care in this population.
Now that the document has been published, there is a need to evaluate the new criteria in prospective studies of athletes of different races, sex, and age participating in distinct sports and at different competition levels. The practical application of these new criteria should help to improve the specificity of ECG interpretation in athletes and reduce false-positive results even further without limiting the ability of the test to detect heart disease.
Although adequate training and experience are necessary, the successive revisions to ECG interpretation criteria (European Society of Cardiology 2010,3 Seattle 20134–7, the refined criteria from 2014,8,12 and the international criteria of 20179–11) have undoubtedly made the ECG an essential, and widely accessible, tool for evaluating athletes.
Conflicts of InterestNone declared.