The guidelines of the European Society of Cardiology (ESC) are endorsed by the Spanish Society of Cardiology (Sociedad Española de Cardiología [SEC]) and Spanish translations are published in Revista Española de Cardiología. Each new guideline is accompanied by a comment, in line with the objectives and methodology recommended in the article published by the Clinical Practice Guidelines Committee of the SEC.1–3 The present article provides a comment on the new ESC guidelines on the assessment of patients undergoing noncardiac surgery.4 Subsequently, the Clinical Cardiology, Geriatric Cardiology, Ischemic Heart Disease and Acute Cardiology Care, and Heart Failure sections of the SEC appointed other experts who have substantially contributed to the present document.
The publication of an updated guideline on this topic is timely, not so much because of new evidence but more because the indication of beta-blockade in noncardiac surgery has had to be revised in depth due to the exclusion of the results of the DECREASE family of trials-which provided the evidence forming the basis of this indication-because of reasonable doubts on their lack of reliability.5
Another interesting point is that, in previous guidelines,6 The European Society of Anesthesiology only supported and endorsed the contents, but has actively participated in the design of the current guidelines. This document emphasizes the central role of anesthesiologists, who should coordinate patient assessment prior to noncardiac surgery.
In other guidelines, the evidence is based primarily on expert opinion. This is especially true in the 2014 guideline. In all, 57% of the recommendations are based on level C evidence. Another important point is that, of the 9 recommendations with level A evidence, 8 are based on clinical trials performed in contexts other than noncardiac surgery. The same is true of 11 of the 43 recommendations with level B evidence. Therefore, many recommendations have been extrapolated from other clinical situations and have never been studied in noncardiac surgery patients. There is no expectation that randomized studies will be performed to support the evidence.
Each of the sections assessed in the new guideline will be discussed below. Emphasis is placed on novel contributions, positive and debatable issues, and implications for clinical practice.
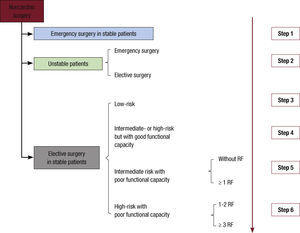
Preoperative AssessmentThe 2014 guideline clarifies the central role of anesthesiology and proposes that a multidisciplinary team carry out cardiovascular assessment in patients whose heart disease could lead to potential complications during noncardiac surgery. These patients are identified as follows: a) patients with known or suspected heart disease that carries a potential perioperative risk; b) those whose perioperative risk could be reduced by optimizing treatment during low- or intermediate-risk surgery, and c) those with known heart disease or a high risk of its development scheduled to undergo high- risk surgery. However, the guidelines make no mention of the importance of physical examination in all preoperative assessments (Figure 1).
Preoperative assessment before noncardiac surgery. Unstable patients are defined as those with unstable angina or a recent myocardial infarction with residual ischemia or significant arrhythmias, or symptomatic valvular disease. Stable patients are defined as those with none of these conditions. RF: risk factors (ischemic heart disease, heart failure, stroke, kidney dysfunction with creatinine > 2 mg/dL, and diabetes mellitus type 1). Step 1: proceed to surgery, maintaining beta-blockade and statin therapy, if started. Step 2: in emergency surgery, urgent revascularization may be advisable, if clinically indicated (II aC); if surgery if elective, ECG (I C), echocardiography (I C), and coronary revascularization may be advisable, if clinically indicated (I A). Step 3: if the patient has no risk factors, proceed to surgery; if the patient has 1 or more risk factors, an ECG can reasonably be performed (IIb C) and in coronary patients statins could be initiated previously (IIa B) and, more questionably, beta-blockers (IIb B). Step 4: procedure to surgery; in coronary patients, statins may be started previously (IIaB) and more questionably beta-blockers (IIbB). Step 5: if the patient has no risk factors, proceed to surgery; ECG can reasonably be performed (IIb C), and if the patient has coronary disease, statins can be started previously (IIa B) and more questionably beta-blockers (IIb B); if the patient has 1 or more risk factors, an ECG is mandatory (I C), and if the patient has coronary disease, statins may be started previously (IIa B) and more questionably beta-blockers (IIb B); ischemia testing is questionable (IIb C). Step 6: if the patient has 1 or 2 risk factors, ECG is mandatory (I C), while echocardiography is questionable (IIb C), as are imaging tests for ischemia imaging (IIb B); if the patient has more than 2 risk factors, ECG is mandatory (I C), as is imaging testing for ischemia (I C) while EGC use is questionable (IIb C); in coronary patients, statins can be started before surgery (IIa B) while preoperative beta-blockade is more questionable (IIb B); coronary revascularization surgery before noncardiac surgery is questionable (IIb B).
The new classification based on surgical risk contains a higher number of high-risk procedures. Thus, these procedures include not only vascular surgery (considered high-risk in the previous guideline) but also liver and lung transplantation, pneumonectomy, and various gastrointestinal and urological procedures, among others.
The decision on the type of surgery to be performed should be based on individualized choice, bearing in mind that less invasive techniques such as endovascular procedures can reduce the immediate surgical risk but are associated with a higher likelihood of future recurrence. A novelty is that the guideline specifically discusses the role of endovascular techniques in abdominal aortic disease. Specifically, both endovascular and surgical treatment of abdominal aortic aneurysms > 55mm is recommended (recommendation IA) if anatomically appropriate for percutaneous therapy and the surgical risk is acceptable.
There is an interesting comment on laparoscopic surgery, which, despite certain initial advantages, has shown no differences with open surgery in the available studies, probably due to the pneumoperitoneum associated with laparoscopy.
Risk stratification is based on 2 considerations: a) functional capacity: as already clear in the previous guidelines, if functional capacity is good, the prognosis is excellent, even though the patient may have coronary disease or multiple risk factors, and b) risk indexes: the guideline discusses 2: the Lee risk index—already proposed in previous guidelines and based on the type of surgery and on 5 clinical variables (ischemic heart disease, heart failure, cerebrovascular disease, renal insufficiency, and diabetes mellitus)— and a new model, the NSQIP MICA, which is less intuitive but can be measured with an online calculator. This index includes the following variables: the type of surgery, the patient's functional status, plasma creatinine, ASA class (5-category classification of the American Society of Anesthesiologists), and age. The model has an interactive index,7 which provides an estimate based on probability models for each individual patient and expresses the results in risk percentage and percentiles. Both indexes have limitations. The Lee risk index was obtained in a cohort consisting primarily of patients undergoing orthopedic surgery. Consequently, the NSQIP model provides better results than the Lee index in general and especially in high-risk patients.8 The authors of the present document have doubts, therefore, about which model should be used and whether prognostic assessment would be improved by using both indexes together rather than separately. Moreover, other factors not included in these risk scales, such as frailty, very low or very high body mass index, anemia and immune status, among others, could interact with the underlying cardiovascular disease and risk factors, which would favor the development of cardiovascular complications after noncardiac surgery.
A commendable feature of the new guidelines is their caution about the use of noninvasive tests and coronary angiography due to the lack of evidence. The number of indications for ECG has decreased, as has their level of evidence compared with previous guidelines. This procedure is not systematically indicated and its use is only advised in patients with risk factors undergoing intermediate- or high-risk surgery (level of evidence: C). Echocardiography is not especially useful in this context, but could be evaluated in patients undergoing high-risk surgery (class IIb recommendation). The guidelines recommends the use of tests for ischemic assessment only in patients undergoing high-risk surgery and with poor functional capacity (< 4 MET, ie, unable to climb 2 flights of stairs or run a short distance), more than 2 risk factors (those mentioned in the paragraph above). For stable patients, coronary angiography can be considered only in those undergoing endarterectomy (class IIb recommendation). In summary, the new guideline emphasizes simple clinical stratification, based on functional capacity and clinical variables, in preference to biomarkers and diagnostic and prognostic tests.
The correct application of this guideline should serve to relieve overburdened cardiology departments in Spain. Firstly, the document acknowledges the central role of anesthesiologists in the preoperative assessment of high-risk patients and these specialists should also coordinate the multidisciplinary team. Secondly, there are fewer indications for ECG and echocardiography, which have traditionally been overused in noncardiac surgery.
Risk Reduction StrategiesRisk reduction strategies include drug use (Table 1), the perioperative management of patients under antiplatelet or anticoagulant therapy, and revascularization.
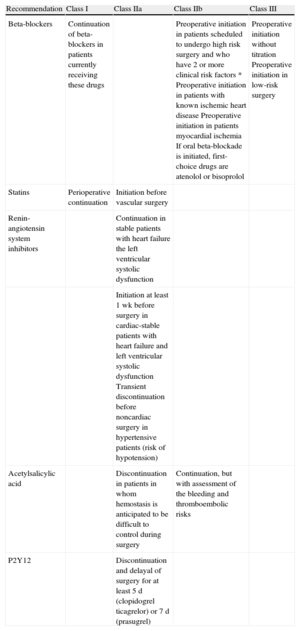
Recommendations and Classes of Recommendation in the New Guideline on the Use of Drugs to Reduce Perioperative Risk
| Recommendation | Class I | Class IIa | Class IIb | Class III |
| Beta-blockers | Continuation of beta-blockers in patients currently receiving these drugs | Preoperative initiation in patients scheduled to undergo high risk surgery and who have 2 or more clinical risk factors * Preoperative initiation in patients with known ischemic heart disease Preoperative initiation in patients myocardial ischemia If oral beta-blockade is initiated, first-choice drugs are atenolol or bisoprolol | Preoperative initiation without titration Preoperative initiation in low-risk surgery | |
| Statins | Perioperative continuation | Initiation before vascular surgery | ||
| Renin-angiotensin system inhibitors | Continuation in stable patients with heart failure the left ventricular systolic dysfunction | |||
| Initiation at least 1 wk before surgery in cardiac-stable patients with heart failure and left ventricular systolic dysfunction Transient discontinuation before noncardiac surgery in hypertensive patients (risk of hypotension) | ||||
| Acetylsalicylic acid | Discontinuation in patients in whom hemostasis is anticipated to be difficult to control during surgery | Continuation, but with assessment of the bleeding and thromboembolic risks | ||
| P2Y12 | Discontinuation and delayal of surgery for at least 5 d (clopidogrel ticagrelor) or 7 d (prasugrel) | |||
*Ischemic heart disease, heart failure, cerebrovascular disease, renal insufficiency, and diabetes mellitus.
As previously mentioned, this guideline incorporates several important novelties in this section, since it provides a response to the controversy aroused by doubts on the reliability of the results of some of the DECREASE family due to questionable methodological procedures, headed by the coordinator of the previous guidelines. These trials substantially contributed to the evidence on the effect of beta-blockade on the results of noncardiac surgery, as they were the only trials observing a reduction in cardiovascular mortality and a tendency to decreased total mortality associated with the use of beta- blockers.9 Another positive feature of the 2014 guideline is the presence of recommendations not included in previous guidelines on the management of patients under dual antiplatelet therapy after coronary stent implantation and on the new oral anticoagulants.
The recommendations on drug use are substantially modified, especially those on beta-blockade. Thus, the only unequivocal indication (class I recommendation) for beta-blockers is their continuation in the perioperative period in patients already receiving this therapy. In previous guidelines, class I recommendations included preoperative instauration of beta-blockers for patients with known ischemic heart disease or evidence of ischemia in a preoperative stress test and systematic administration in patients scheduled to undergo high-risk surgery. These recommendations have been relegated to class IIb. Moreover, treatment instauration before a high-risk procedure may be considered for patients with 2 or more clinical risk factors. In these patients, the guideline recommends atenolol or bisoprolol. With both drugs, treatment should be started at low doses with progressive increments.
The use of angiotensin converting-enzyme inhibitors and angiotensin II receptor blockers has been relegated to class IIa recommendation both for continuation therapy and for treatment instauration in stable patients with heart failure and systolic dysfunction.
The recommendations on statin use have been slightly modified. The only class I indication is perioperative continuation, preferably with statins with a long half-life or extended-release formulations. Preoperative statin initiation between 30 days and 1 week before a high-risk intervention (class I recommendation in previous guidelines) is currently restricted to vascular surgery and should ideally be started at least 2 weeks before the procedure (class IIa recommendation).
In previous guidelines, the use of alpha 2 receptor agonists was recommended (class IIb) to reduce perioperative complications in vascular surgery. The unfavorable results of the POISE-2 study with clonidine were decisive for advising against their use in the current guideline.
New recommendations have been added to perioperative management in patients under antiplatelet therapy, with specific considerations for patients receiving dual antiplatelet therapy. Acetylsalicylic acid therapy should not be interrupted for 4 weeks after conventional stent implantation and for 3-12 months after drugeluting stent implantation, depending on the type of stent, unless the risk of life-threatening surgical bleeding is unacceptably high (class I recommendation).
The 2014 guideline confirms the recommendation to discontinue acetylsalicylic acid therapy when it is anticipated that hemostasis will be difficult to control during surgery. Otherwise, the decision to maintain acetylsalicylic acid therapy during the perioperative period, a class IIa recommendation in the previous guideline, is now a class IIb indication and should be an individualized decision weighed against the risk of thrombotic and bleeding complications. Clopidogrel and ticagrelor should be discontinued at least 5 days before surgery and prasugrel at least 7 days before surgery.
The current guideline incorporates novel information on the perioperative management of patients receiving anticoagulants, particularly those other than vitamin K antagonists. The document stresses that the use of heparin for bridging in these patients should be avoided due to the short biological half-life of these oral anticoagulants. Another point that should probably be added is that these agents are not indicated in many of the situations associated with high thromboembolic risk (such as mechanical prostheses and thrombophilia).
There are few changes to recommendations on revascularization. In general, the recommendations are maintained on the timing of noncardiac surgery in patients with prior revascularization. Also maintained are the recommendations on the optimal timing of noncardiac surgery after percutaneous revascularization, but they are now less categorical (previously class I, now class IIa): noncardiac surgery should be performed 3 months after conventional stent implantation and 12 months after drug-eluting stent implantation. The minimum recommended time after conventional stent implantation is reduced to 4 weeks (from 6 in the previous guideline) and to 6 months for patients with new generation drug-eluting stents.
The current document highlights the lack of benefit of systematic prophylactic myocardial revascularization for asymptomatic patients with stable coronary disease, although (as in the previous guideline) prophylactic myocardial revascularization may be performed before high-risk surgery, depending on the extent of a stress-induced perfusion defect (class IIb recommendation). With regard to the type of myocardial revascularization, the document raises the possibility of extending the use of new-generation drug-eluting stents, which require a considerably shorter period of dual antiplatelet therapy, if the results of preliminary studies are confirmed, both in patients with stable ischemic heart disease and in those with non-ST-segment elevation acute coronary syndrome. In these patients, if noncardiac surgery is needed for severe disease and myocardial revascularization, an expert team should discuss the priority of surgery (the previous guideline gave priority to surgery).
An issue that remains to be definitively settled is the advisability of starting or maintaining acetylsalicylic acid therapy in situations with an unequivocal indication, such as chronic stable ischemic heart disease, beyond the safety periods after coronary stent implantation. The guideline recommends the difficult task of weighing up the risk of bleeding or thrombotic complications to adopt an individualized decision, despite the lack of objective tools to measure these risks in this context.
The main message is the recommendation to continue the administration of drugs conferring demonstrated cardiovascular protection (beta-blockers, renin-angiotensin system inhibitors, statins) during the perioperative period in patients taking these drugs appropriately and chronically and who remain stable. Starting treatment, particularly beta-blockade, shortly before the intervention could be harmful, since its adverse effects may outweigh its potential benefits. If treatment is necessary, it should be prescribed cautiously and sufficiently in advance of the intervention (statins at least 14 days before, renin-angiotensin system inhibitors at least 7 days before, and beta-blockers at least 2 days before) to allow adequate treatment monitoring.
Specific DiseasesThe guideline devotes broad information on increasingly frequent clinical entities, such as heart failure, aortic valve stenosis, and chronic kidney disease, but does not mention specific heart diseases, such as hypertrophic cardiomyopathy. Unfortunately, there is little evidence on this topic, and consequently many recommendations are based on expert opinion and are therefore open to debate.
In heart failure, left ventricular function should be evaluated by echocardiography or natriuretic peptides (class IA recommendation), unless recently performed, in patients with established or suspected heart failure awaiting intermediate- or high-risk surgery. The indication for echocardiography is undoubted in patients with clinical suspicion of heart failure without prior evaluation of ventricular function and in those with an established diagnosis whose condition worsens, but is less well established in stable patients with known heart failure under optimal therapy. The recommendation on the use of natriuretic peptides should also be applied with caution, due to the lack of definitive evidence on their usefulness in the management of these patients. The new class I recommendations on the pharmacological treatment of heart disease are based on those established in the previous section: preoperative optimization of drug therapy with beta- blockers, renin-angiotensin system inhibitors, aldosterone antagonists, and diuretics; if feasible, intermediate- and high-risk surgery should be delayed for at least 3 months since the start of treatment in patients with a new diagnosis of heart failure, and discontinuation of renin- angiotensin system inhibitors on the day of surgery should be assessed, depending on the patient's blood pressure.
Recommendations on valvular heart disease focus on aortic stenosis. Surgical valve replacement before noncardiac surgery is recommended in patients with severe symptomatic aortic stenosis, unless the valvular surgery is high risk (class I recommendation). In high-risk valvular surgery, transcatheter aortic valve implantation (TAVI) or balloon aortic valvuloplasty should be considered (class IIA). It is surprising that this indication is not in agreement with that given in the ESC guideline on valvular heart disease published in 2012,10 which recommends noncardiac surgery with strict monitoring in patients with severe symptomatic aortic stenosis and a high-risk of an adverse outcome of valvular surgery. Lastly, in patients with severe asymptomatic aortic stenosis scheduled to undergo high-risk noncardiac surgery, surgical valve replacement is also recommended, unless there is a high risk of an adverse outcome of the valvular surgery (class IIa). This recommendation is also controversial and it may be reasonable to perform noncardiac surgery without prior valve replacement in these patients.
There are few changes to recommendations in patients with mitral stenosis and mitral regurgitation. The new guidelines add a paragraph on secondary mitral regurgitation but make no specific recommendations. Noncardiac surgery may be considered in patients with severe mitral regurgitation who do not have symptoms or left ventricular dysfunction, and percutaneous mitral commissurotomy should be considered in patients with severe symptomatic or asymptomatic mitral stenosis and pulmonary artery systolic pressure above 50mmHg scheduled to undergo intermediate- or high-risk noncardiac surgery (class IIa recommendation).
The 2014 guideline makes new recommendations on measures to reduce contrast-induced renal injury. Detailed recommendations are made on the management of patients with contrast media-induced kidney disease (class I recommendation) concerning the use of hydration with saline serum before contrast administration, the use of isoosmolar or low osmolarity contrast media, and contrast volume reduction. Among these specific recommendations for renal protection against contrast-induced injury, a notable feature is that the guidelines include short-term high-dose statin therapy (class IIa recommendation), based on a single clinical trial with a small number of patients in a specific clinical context and with a debatable study design.
The section on pulmonary disease contains new recommendations, especially those related to the treatment of pulmonary arterial hypertension (6 class I recommendations) and the recommendation that all smokers quit smoking at least 2 months before noncardiac surgery (class I recommendation).
Perioperative MonitoringIn this section, the 2014 recommendations depart little from those of previous guidelines, all of which had a low level of evidence (1 with level A, 2 with level B, and 8 with level C). The use of ECG monitoring for the detection of arrhythmias and myocardial ischemia continues to be mandatory for any type of surgery requiring anesthesia, whether general or regional. Transesophageal echocardiography (TOE) for wall motion abnormalities is not systematically indicated in patients undergoing noncardiac surgery due to the low frequency of these abnormalities in these patients and its low prognostic value in the postoperative period. In contrast, intraoperative TOE monitoring should be used in patients who develop hemodynamic instability (class I recommendation), in those showing changes on intraoperative on ECG monitoring (class IIa), and in patients undergoing high-risk noncardiac surgery and who also have a high risk of ischemic or hemodynamic disturbances or severe valvular lesions. A novelty is that the new guideline recommends intraoperative transesophageal Doppler to monitor cardiac output and to guide fluid optimization.
Natriuretic peptides and high-sensitivity troponin have been systematically used after noncardiac surgery in many centers. Based on meta-analyses and prospective studies, the 2014 guideline recommends that measurement of natriuretic peptides and high- sensitivity troponin after surgery can be considered in high-risk patients to improve risk stratification (IIb B recommendation). The patients who could derive the most benefit from determination of these biomarkers are those with functional capacity < 4 METs and a “revised cardiac index” or Lee index < 1 (for vascular surgery) and > 2 (for nonvascular surgery). These recommendations can be considered as the first step toward protocolizing biomarker use in the postoperative period after noncardiac surgery.
An important feature of the 2014 guideline relates to glycemia monitoring during surgery. Several recommendations are made in this section, given the paramount importance of diabetes mellitus and glucose metabolism alterations as predictors of morbidity and mortality during and after noncardiac surgery. The role of strict glycemic control is unclear in nondicabetic patients undergoing noncardiac surgery. Regarding strict glycemic control (81-108 mg/dL), the guidelines advise caution against extrapolating the results of early studies in nonsurgical patients in the critical care setting to those undergoing noncardiac surgery. The results from early studies have been refuted due to recent reviews showing that better outcomes are associated with less strict glycemic control (140-180 mg/dL).11 Consequently, the guideline assigns a class IB recommendation to intensive hyperglycemia management in the postoperative period (with intravenous insulin therapy), setting the trigger for instigating intravenous insulin therapy at 180 mg/dL but maintaining glucose levels above 110 mg/dL, due to the potential risk of hypoglycemias associated with strict glycemic control strategies. The latter specification is a new addition, since previous guidelines stressed only the need to avoid hypoglycemia, but without establishing a lower safety limit.12 An important novelty is the inclusion of preoperative screening for elevated glycosylated hemoglobin (HBA1c) before high-risk major surgery, although this recommendation is based on expert opinion (IIaC). The introduction of HBA1c in the perioperative analytic profile does not represent a large cost increase, given that high-risk procedures account for a small percentage of the total number of surgical interventions carried out in Spain.13
Finally, a new section on anemia stresses its contribution to the development of ischemia in patients with preexisting ischemic heart disease. However, the guideline makes no specific recommendations and merely states that transfusion, if required, should be given according to clinical needs. This recommendation contrasts slightly with the more precise indications of the United States guidelines.14
AnesthesiaThe new guideline enters into the topic of anesthesia in noncardiac surgery in greater depth but without providing specific recommendations on the type of anesthetic agent that should be used (inhaled or intravenous), specifying that the evidence favoring inhaled anesthetics such as sevoflurane are derived from studies in cardiac surgery patients.
The guideline makes an important observation on safe blood pressure values during anesthesia induction and maintenance to avoid postoperative complications. Both hypotension (mean < 60 mmHg) and deep sedation defined as a Bispectral Index Scale score < 45 for more than 30minutes should be prevented.
Neuroaxial anesthesia may be considered to reduce the perioperative morbidity and mortality associated with general anesthesia (IIb B recommendation). Because neuroaxial anesthesia is a form of regional anesthesia, there is strong debate about its benefits vs those of general anesthesia. However, according to the guideline, this form of anesthesia may be considered in patients at high surgical risk (with cardiovascular risk factors or established cardiovascular disease) and without contraindications to avoid peri- and postoperative complications.
Another addition is the paragraph on goal-directed fluid therapy, which has been added due to the development of advanced intraoperative cardiac monitoring techniques and algorithms that simplify volemia estimation and response to fluid therapy to correct hemodynamic instability during surgery. The use of increasingly less invasive techniques, such as transpulmonary dilution, and techniques derived from advanced pressure waveform analysis have allowed this type of therapeutic approach to gain ground over previously used techniques, based mainly on pulmonary catheters and thermodilution methods. Therefore, the guideline assigns a class IIa B recommendation to the use of these goal-directed fluid strategies in patients at high surgical or cardiac risk (cardiomyopathies, valvular disease, etc).
Postoperative pain leads to increased complications and sympathetic tone, slower recovery, and patient distress. A range of options is available for pain control, such as neuroaxial analgesia, local anesthesia, opioids, and nonsteroidal anti-inflammatory agents. Compared with previous guidelines, the current document provides greater detail on analgesia. Neuroaxial analgesia should be used with caution and bearing in mind the risk-benefit ratio in each patient. Nonsteroidal anti-inflammatory agents may now be considered (previously the contraindication was absolute).
Conflicts of InterestNone declared.
Working Group of the SEC for the 2014 ESC/ESA Guidelines on non-cardiac surgery: cardiovascular assessment and management: José Alberto San Román (coordinator), Alfredo Bardají (coordinator), Manuel Almendro, Teresa Blasco, Vicente Bodí, Juan Antonio Castillo, Juan José Gómez Doblas and Carlos González Juanatey.
Expert Reviewers for the 2014 ESC/ESA Guidelines on non- cardiac surgery: cardiovascular assessment and management: Pilar Carrillo, Manuel Martínez-Sellés, Rosa González Davia, Javier López Díaz, Alessandro Sionis Green, Rosa María Lidón Corbi, Javier Segovia Cubero, Antonio Fernández Ortiz, Ramón Bover, Domingo Marzal, Juan Cosín Sales, and Lorenzo Fácila.
Clinical Practice Guidelines Committee of the SEC: Manuel Anguita Sánchez (President), Ángel Cequier Fillat (Secretary), Lina Badimón Maestro, José Antonio Barrabés Riu, Josep Comín Colet, Ignacio Fernández Lozano, José Juan Gómez de Diego, Manuel Pan Alvarez-Osorio, Luis Rodríguez Padial, José Alberto San Román Calvar, and Pedro Luis Sánchez Fernández.