Since the publication of the 2015 European Society of Cardiology (ESC) guidelines for the management of endocarditis, important new data have been published mandating an update of recommendations, which are synthetized in these current 2023 ESC guidelines.1,2 This article, prepared by a group of experts proposed by the Guidelines Committee of the Spanish Society of Cardiology, aims to highlight the most relevant novelties, the consequences of their implementation in our setting, and the gaps in evidence in order to improve local clinical practice.
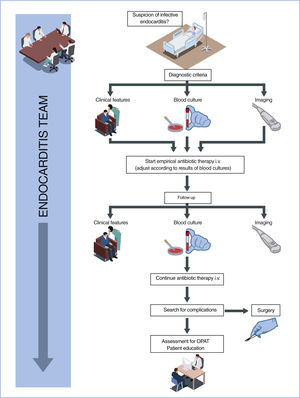
WHAT IS NEW?The guidelines enhance the value of the “endocarditis team”, upgrading the same 2 previous recommendations to IB: the need for management of complicated infective endocarditis (IE) in a referral center with an endocarditis team and immediate surgical facilities, and the need for early and regular contact with such a center in cases of uncomplicated IE (figure 1).
This section is one of the most novel aspects of these guidelines compared with the 2015 guidelines. The ESC 2023 guidelines, while recognizing the absence of evidence, have broadened their recommendations, assuming the severity of the disease and the very small incidence of serious adverse reactions following the administration of a single antibiotic dose. In summary, the main changes are as follows:
- •
Strengthened class of recommendation from IIa to I in patients at high-risk of IE undergoing oro-dental procedures (evidence level B for previous IE and C for valvular prostheses carriers, including transcatheter prostheses, and complex congenital heart disease).
- •
Added indication IIaC for patients with transcatheter mitral or tricuspid repair procedures, IC in patients with ventricular assist devices and IIbC in heart transplant patients with dental procedures with a high-risk of IE.
- •
Reintroduction of the possibility of systemic antibiotic prophylaxis in invasive procedures of the respiratory, gastrointestinal, or genitourinary tract, musculoskeletal system, and skin, with a low class of recommendation (IIbC).
- •
The use of clindamycin in patients allergic to beta-lactams is discouraged, due to the increased risk of Clostridium difficile infections, replacing it with macrolides or doxycycline.
- •
A perioperative prophylactic antibiotic is recommended for any valve prosthesis implantation procedures (IB), electronic devices (IA), and occluders or grafts (IB). The recommended antibiotic is cefazolin 1g IV for implantation of electronic devices and amoxicillin/clavulanic 2g or ampicillin 3g for transcatheter prosthesis implantation.
The diagnosis of IE is based on clinical suspicion, supported by microbiological data and imaging techniques. Transthoracic (TTE) and transesophageal echocardiography (TOE) are the cornerstone diagnostic imaging techniques, but a multimodality imaging approach is now strongly encouraged. TOE is now a class I recommendation, even in cases with positive TTE, except in isolated right-sided native valve IE with good quality TTE examination and unequivocal findings. Performing an echocardiogram should be considered not only in patients with S. aureus but also in those with E. faecalis, and some Streptoccocus spp bacteraemia (class IIa). Regarding follow-up, TOE is now recommended when the patient is stable before switching from intravenous to oral antibiotic therapy (class I). Recommendations on the role of diagnostic alternatives such as computed tomography (CT), magnetic resonance imaging (MRI) and nuclear imaging, have been more clearly described and upgraded in the presence of prosthetic valves or cardiovascular implanted electronic devices (CIED). The guidelines highlight their role in the diagnosis of IE and detection of cardiac complications, distant lesions, and sources of bacteremia. Specifically, cardiac CT angiography is encouraged in patients with “possible” native valve endocarditis to detect valvular lesions and confirm diagnosis, and [18F]FDG-PET/CT or CT angiography in cases of “possible” prosthetic valve endocarditis (PVE) to detect valvular lesions and confirm diagnosis.3 Brain and whole body imaging (CT, [18F]FDG-PET/CT, and/or MRI) is also recommended in patients with symptoms suggesting embolic complications to detect peripheral lesions or add minor diagnostic criteria (class IB), and may be performed in asymptomatic patients for screening of peripheral lesions (class IIb). New clear algorithms for the diagnosis of native-valve, prosthetic-valve and CIED-associated IE are included.
Changes have been made to the diagnostic criteria. With regards to major criteria, the presence of imaging positive for IE has been simplified, including any valvular, perivalvular/periprosthetic and foreign material anatomic and metabolic lesions characteristic of IE detected by echocardiography, CT, [18F]-FDG-PET/CT(A), or white blood cell (WBC) SPECT/CT. Abnormal prosthetic or periprosthetic uptake detected by [18F]FDG-PET/CT or WBC SPECT/CT should be considered a major criterion for PVE, irrespective of the interval from surgery. E. faecalis has been included in the typical organisms. Minor criteria include frequent embolic vascular dissemination, even asymptomatic lesions detected by imaging only.
Antimicrobial therapyThe panel recognizes that there is insufficient evidence to establish that, in IE, any one antibiotic regimen is superior to another.4 While there is a consensus on the antimicrobial treatment of streptococcal and Enterococcus faecalis IE, the best treatment of staphylococcal prosthetic IE, especially if caused by methicillin-resistant strains, is unknown. A word of caution is issued regarding possible interactions and toxicities related to commonly used drugs (rifampicin, gentamicin), as well as “new” drugs (daptomycin, fosfomycin). Not only cloxacillin, as in the previous guidelines, but also cefazolin are considered drugs of first choice for the treatment of staphylococcal endocarditis caused by methicillin-susceptible strains. The safety and efficacy of outpatient parenteral or oral antibiotic treatment have changed the paradigm of antimicrobial therapy in stable IE patients.5 Thus, treatment is divided into 2 periods: an initial phase (early critical phase) in which infection control and patient stabilization must be guaranteed, and a second phase (continuation phase with resting bacteria) in which the objective is to complete antibiotics, if possible, orally and at home.
SurgeryAs in the previous guidelines, the new guidelines focus on the 3 main reasons for early surgical indication in the setting of IE: heart failure, uncontrolled infection, and septic embolization prevention. A new clear classification of timing definitions of surgery is proposed. The task force has defined emergency surgery (<24hours irrespective of preoperative duration of antibiotic treatment), urgent surgery (3-5 days), and nonurgent surgery (during hospital admission). The time of urgent surgery is shortened from <7 days to 3 to 5 days, highlighting that unnecessary delays should be avoided once the indication for urgent surgery is established, with an important clinical implication, particularly in nonreferral centers.
There are no differences in the indications for surgery in patients with heart failure, the main indication for urgent and emergency surgery for IE. In the section on uncontrolled infection, small changes are introduced. The table of recommendations specifically indicates that prosthetic dehiscence and new atrioventricular block are indications for urgent surgery. Moreover, urgent surgery should be considered in IE with positive blood cultures >1 week and adequate control of metastatic foci. In this regard, it is enough to demonstrate positive blood cultures 2 to 3 days after starting the correct antibiotic treatment for this recommendation.6
The guidelines propose a more liberal use of surgery to prevent embolisms. A recommendation of urgent surgery in IE with vegetation ≥10mm and other indications for surgery have been upgraded to class I. In addition, urgent surgery may be considered in aortic or mitral IE with vegetation ≥10mm and without severe valve dysfunction or without clinical evidence of embolism and low surgical risk (class IIb). The analysis of vegetations and the clinical characteristics by the endocarditis team is essential for clinical management and optimal early surgical decision: a deeper characterization of imaging features of vegetations (size, shape, movement patterns, etc) could help to discriminate between dangerous lesions potentially leading to embolic events, and low-risk vegetations.7 Finally, the guidelines indicate the need for an adequate assessment of operative risk using different risk scores designed specifically for the setting of IE.
After a stroke, in patients with strong surgical indications, operative management is recommended (class IB) without delay (provided there is absence of severe neurological injury, coma, or intracranial bleeding). For stable patients with intracranial bleeding, delaying surgery (>1 month) is still recommended with repeat assessment of clinical and imaging stability. In patients with unstable features that would lead to the need for earlier intervention to allow survival, the guidelines include the possibility of proceeding with surgery provided that meaningful neurological outcome is likely (IIaC). Regarding right-sided IE, the guidelines upgrade the indications for surgery (repair over prosthetic replacement): right-sided dysfunction secondary to severe tricuspid regurgitation without diuretic refractoriness (class IB), residual vegetations >20mm after pulmonary embolism (class IC), simultaneous left-sided involvement (class IC), and persistent vegetation with severe respiratory failure after pulmonary embolism (class IB). Finally, the guidelines provide insight into the choice of prosthetic valve for mitral or aortic valve replacement, with a liberal use of bioprosthetic replacement material as disease complexity increases.
Complications and specific situationsThe guidelines describe other complications of IE and their clinical management. Neurological complications of IE are associated with excess morbidity and mortality, and prompt diagnosis of IE and early initiation of antibiotic therapy are essential to prevention. In infective cerebral aneurysms with an indication for interventional treatment, endovascular therapy should be proposed. Epicardial pacemaker implantation should be considered in patients undergoing surgery for IE and complete atrioventricular block, if one of the following predictors of persistent atrioventricular block is present: preoperative conduction abnormality (prolonged PR and QRS intervals), S. aureus infection, aortic root abscess, tricuspid valve involvement, or previous valvular surgery. The guidelines provide precise criteria for diagnosing and managing CIED–related IE, and [18F] FDG-PET/CT(A) may be considered in possible CIED-related IE to confirm the diagnosis of IE. Complete system extraction without delay is recommended in patients with definite CIED-related IE under initial empirical antibiotic therapy. In all cases, general prevention measures must be followed in patients with CIEDs. Finally, if there are musculoskeletal manifestations, imaging techniques (MRI or PET/CT) are recommended in patients with suspected spondylodiscitis and vertebral osteomyelitis complicating IE.
Discharge and follow-upAfter hospital discharge, to detect the appearance of possible subsequent complications, 2 follow-up periods are distinguished: the first year after IE, and long-term prognosis. The risk of recurrence (which includes relapses and reinfections) has increased and ranges between 2% and 9%. The same description and management are maintained for the term “relapse” and “reinfection” and, as a novelty, an algorithm differentiates these 2 clinical situations. New recommendations for follow-up include psychosocial support for the patient and family, detection of anxiety and depression, referral for psychological treatment, if necessary, addiction treatment in intravenous drugs addicts, and cardiac rehabilitation in stable patients at least 2 weeks after left-sided IE surgery. Health education for patients and caregivers remains essential during this period.
CONSEQUENCES OF ITS IMPLEMENTATION IN OUR SETTINGRegarding prevention of IE, the main consequence of its implementation will be an increase in antibiotic prophylaxis and its extension to intermediate risk situations and procedures other than oro-dental procedures. The few studies carried out in Spain on the follow-up of the previous guidelines already indicated an “overindication” of prophylaxis in these now accepted cases,8,9 and therefore the new guidelines seem to come closer to the reality of our setting.
Echocardiography is widely accessible; however, a wider use of TOE is anticipated as it is now a key diagnostic modality not only for diagnosis, but also before switching to oral therapy. This is even more challenging in the context of newer imaging modalities that are now strongly recommended but may not be accessible in all centers, which may translate into the need for diagnosis/treatment in specific centers. The number of studies, and therefore their associated cost, will increase due to the new recommendations.
The range of antibiotic treatment options for staphylococcal prosthetic IE can lead to some confusion. Nevertheless, this range allows each center to adapt its protocol to its context and drug availability. The preferential use of cefazolin is expected to reduce the risk of interstitial nephritis and phlebitis associated with the use of cloxacillin. Facilitating early discharge to home once the septic process has been resolved has significant benefits for the patient in terms of quality of life and a reduced risk of complications. However, this requires an agile diagnostic and therapeutic process at the referral center, an adequate infrastructure for home follow-up, and a great deal of coordination between the various hospital departments and primary care.
Considering that most patients requiring surgery during the active phase fall into the category of urgent surgery, the change in the definition of timing will immediately lead to earlier intervention. Most cardiovascular surgery departments have imposed limitations on daily surgical slots and intensive care unit beds. There is a need to evaluate whether the adoption of the recommendation of these new guidelines will be possible through an increased flexibility in surgical scheduling and enhanced local resource support. The need for tertiary hospitals to be able to respond in a timely manner to IE cases resembles to some extent the former challenges faced in the process of improving door-to-balloon time for acute myocardial infarction management. The most liberal indication for surgery to prevent embolisms is based on a few nonrandomized studies and weak evidence and should be formally evaluated before being included in our daily practice.
In patients with recent stroke, the risk of perioperative neurological exacerbation must be balanced against that of delaying heart surgery. Although there is little evidence, a liberal push toward earlier surgery after ischemic stroke is reflected in the new recommendations. Perioperative management for patients after stroke is a challenging endeavor that requires an experienced operating team. Tailored pre- and postoperative management of these patients will greatly influence the possibility of obtaining the benefits of an early intervention. Successful outcomes that support the current aggressive recommendations arise usually from centers of excellence.10 The decision to implement nondelayed surgical management after stroke in less experienced environments needs to be adapted locally considering that the body of evidence remains small.
Complete system extraction without delay is recommended in patients with definite CIED-related IE under initial empirical antibiotic therapy. Percutaneous rather than surgical extraction is the preferred procedure but requires specialized tools and should be performed in centers with expertise in this technique and with on-site surgical backup, due to the risk of life-threatening tamponade and vein laceration. To comply with these recommendations, the creation of referral centers is mandatory.
In our setting, the recommendation for cardiac rehabilitation programs can be difficult to implement due to the insufficient availability of these units and territorial variability.
GAPSThe guidelines address almost all situations and patients at risk for IE, thus providing very comprehensive guidelines. The main gap, in general, is the lack of strong evidence for almost all situations, which is reflected in a majority of level of evidence C. There is uncertainty regarding whether echocardiography should be systematically performed in patients with bloodstream infections or if there are strategies that allow the identification of patients at higher risk of IE. Are scoring systems trying to overcome these uncertainties? The absence of a need for a time interval after surgery for the performance of PET/CT will probably be further studied in coming years. In the current guidelines, there remains a lack of evidence to recommend the best treatment (ie, the most effective and least toxic) for staphylococcal prosthetic IE. More real-life data on oral treatment are needed to support the current recommendations. The evidence to support immediate surgery after stroke is limited to observational studies. Thus, despite the upgraded recommendation for immediate surgery after stroke, scrutiny of the impact of this liberal implementation is required. There are no recommendations for the new devices, and no comments on how to manage cases of suspected IE in leadless pacemakers or whether patients with a subcutaneous defibrillator require special management. The in-hospital mortality rate of patients with IE has remained high and unchanged over the past 2 decades, but the identification of new markers of high risk may offer the opportunity to change the course of the disease. Several surgical risk scoring systems have been developed but none are used in routine clinical practice. Prospective surgical scoring systems with better precision need to be developed, particularly to help determine surgical futility in high-risk patients.
CONCLUSIONSThe new guidelines for the management of endocarditis update new evidence on prophylaxis, diagnosis, medical, and surgical treatment. They provide practical figures and algorithms, which help physicians to make simple decisions in daily clinical practice. The guidelines also highlight the need for a multidisciplinary approach, and the key-role of the endocarditis team in the assessment of high-risk patients and the decision-making process.
FUNDINGNone.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCENo artificial intelligence has been used in the preparation of this article.
CONFLICTS OF INTERESTThe conflict-of-interest declaration documents of all authors can be seen in the supplementary data.
SEC Working Group for the 2023 ESC guidelines for the management of endocarditis: David Vivas (coordinator), Alberto San Román (coordinator), Manuel Anguita, Nuria Fernández-Hidalgo, Ignacio Fernández-Lozano, Carlos González-Juanatey, Ariana González, Marta Parellada, Eduard Quintana.
SEC Guidelines Committee: José Luis Ferreiro (president), Pablo Avanzas (secretary), Rut Andrea, Araceli Boraita, David Calvo, Raquel Campuzano, Victoria Delgado, Laura Dos Subirá, Juan José Gómez Doblas, María Antonia Martínez Momblan, Pilar Mazón, Domingo Pascual Figal, Juan Sanchis, José María de la Torre Hernández, David Vivas.
See related article: https://secardiologia.es/cientifico/guias-clinicas/miscelanea/14533-2023-esc-guidelines-for-the-management-of-endocarditis
The names of all the authors of the article are listed in alphabetical order in Appendix A.
Corresponding author.
Email address: dvivas@secardiologia.es.