The lower prevalence of type 2 diabetes mellitus (T2DM) in patients with heterozygous familial hypercholesterolemia (HeFH) could explain why T2DM has not always been identified as an independent predictor of cardiovascular disease (CVD) in different familial hypercholesterolemia cohort studies. The aim of the present study was to evaluate clinical and genetic aspects of HeFH patients with T2DM in the dyslipidemia registry of the Spanish Arteriosclerosis Society.
MethodsHeFH patients were classified according to the presence or absence of T2DM. The clinical, biochemical and genetic characteristics of the 2 groups were compared.
ResultsOf the 2301 patients with primary hypercholesterolemia included in the registry, 1724 with a probable or definite diagnosis according to the Dutch Lipid Clinic Network score were finally included. HeFH patients with T2DM had a higher rate of CVD and a less favorable lipid profile, with higher total cholesterol (366.9±86.7mg/dL vs 342.0±74.7mg/dL; mean difference 24.894; 95%CI, 5.840-43.949) and non–high-density lipoprotein cholesterol (316.9±87.8mg/dL vs 286.4±75.4mg/dL; mean difference 30.500; 95%CI, 11.211-49.790) levels. No significant differences were found between the groups concerning the specific type of HeFH-causing mutation (P=.720). After adjustment for major risk factors, logistic regression analysis confirmed a relationship between T2DM and the presence of CVD (OR, 2.01; 95%CI, 1.18-3.43; P=.010).
ConclusionsHeFH patients with T2DM have a higher rate of CVD and a less favorable lipid profile, regardless of genetic mutation type. In these patients, T2DM is associated with the presence of CVD.
Keywords
Heterozygous familial hypercholesterolemia (HeFH), caused by mutations in genes encoding for the low-density lipoprotein (LDL) receptor, apolipoprotein (apo) B, proprotein convertase subtilisin/kexin type-9 (PCSK9) or apo E,1 leads to elevated LDL cholesterol levels, producing a higher incidence of cardiovascular events.2 These patients are considered at high cardiovascular risk according to the European and American clinical guidelines.3,4 Moreover, the recent guidelines of the American Association of Clinical Endocrinologists and the American College of Endocrinology consider established cardiovascular disease (CVD) and familial hypercholesterolemia (FH) or diabetes to constitute an extreme cardiovascular risk category and recommend an LDL cholesterol level of <55mg/dL as the therapeutic target.5
However, CVD seems to be highly variable in HeFH patients, even in those sharing the same pathogenic mutation.6 In this respect, the International Atherosclerosis Society proposed certain criteria to define a phenotype of patients with severe HeFH7 with a probable increased cardiovascular risk. Risk stratification in HeFH is an important issue; however, these proposed criteria do not seem to solve this problem.8,9 Therefore, it is essential to identify FH patients at higher risk of CVD to allow clinical decision-making on the indication for aggressive lipid-lowering therapies such as PCSK9 inhibitors.
On the other hand, available evidence suggests a decreased risk of type 2 diabetes mellitus (T2DM) in patients with HeFH compared with the general population, although the exact mechanism of this possible protective effect is still not fully understood.10,11 Although T2DM is an independent predictor of CVD in the general population,12,13 this may not be the case in the HeFH population. Hence, while T2DM was an independent cardiovascular risk factor in British Columbia (Canada),14 the Dutch FH cohort15 and the CASCADE-FH registry of the United States16, this finding could not be replicated in other FH cohorts such as those from Quebec (Canada),17 Utah (United States),18 Brazil,19 Australia,20 and Greece.21 In a Spanish registry,22 although T2DM was considered a prognostic factor in the univariate analysis, it was not included in the final analysis, probably due to the low prevalence of impaired glucose metabolism in patients with HeFH.
This may be explained, at least in part, by the low incidence of impaired glucose metabolism in this specific population. By contrast, in a recent meta-analysis and systematic review assessing the association of risk factors and CVD in HeFH patients, smoking, hypertension, and T2DM accounted for more than a fourth of CVD risk in HeFH patients.23
The present study aimed to evaluate the clinical, biochemical and genetic characteristics of HeFH patients with T2DM compared with those without T2DM in the large dyslipidemia registry of the Spanish Arteriosclerosis Society.
METHODSStudy protocolThe dyslipidemia registry of the Spanish Arteriosclerosis Society was created in 2013 as an online registry in which 50 certified lipid units distributed throughout Spain enter cases with different forms of primary hyperlipidemias. These lipid units are the centers in the Spanish National Health Service where most cases of primary hyperlipidemias are referred for clinical management. The registry was approved by a central ethics committee to include anonymous clinical data (Comité Ético de Investigación Clínica de Aragón, Zaragoza, Spain) in accordance with the ethical guidelines of the Declaration of Helsinki. The patients did not have to sign an informed consent form as the data were obtained from an official national registry; however, since September 2018, participants’ written consent has been obtained in accordance with the instructions of the Ethics Committee. Minimum data for the inclusion of cases in the registry are the following: age, sex, smoking status, personal history of hypertension, diabetes and CVD with age at the first event, body mass index, waist circumference, complete lipid profile including total cholesterol, LDL cholesterol, triglycerides and high-density lipoprotein (HDL) cholesterol levels without lipid-lowering treatment at diagnosis, and current lipid and biochemical parameters at the moment of inclusion in the registry. CVD in the registry is defined as coronary heart disease (myocardial infarction, acute coronary syndrome with stenosis> 50% of a main coronary artery and coronary revascularization), stroke (ischemic and hemorrhagic), aortic aneurysm and lower limb ischemia (intermittent claudication with ankle/brachial index <0.90 or revascularization of lower limb arteries). T2DM is defined as fasting blood glucose> 125mg/dL or taking blood-glucose-lowering drug therapy.
In the present study, inclusion criteria were patients aged ≥ 18 years with probable (6-8 points) or definite (> 8 points) HeFH according to the Dutch Lipid Clinic Network criteria,24 and complete minimum data for the registry such as information on family history of hypercholesterolemia and premature CVD, personal history of tendon xanthomas and corneal arch before the age of 45 years, genetic study of LDLR, APOB or PCSK9, age at statin onset and age at T2DM diagnosis.
Patients with HeFH were classified according to the presence or absence of T2DM at the moment of inclusion in the registry. The clinical, biochemical and genetic characteristics of the 2 groups were compared.
Exclusion criteria were diagnosis of homozygous FH and lack of data on family or personal history of CVD, lipid-lowering therapy, age at T2DM onset or absence of genetic analysis.
Biochemical measurementsBlood samples were collected after an overnight fast (> 10 hours) and were processed for laboratory analyses the same day. Serum total cholesterol, triglycerides and HDL cholesterol levels were measured locally using enzymatic methods. Serum LDL cholesterol concentration was calculated using the Friedewald formula.
Genetic analysisDNA was isolated from whole blood using standard methods and screening for LDLR, APOB and PCSK9 mutations was carried out using the Lipochip Platform (Progenika Biopharma S. A., Bilbao, Spain). This specific platform consists of 2 consecutive steps: the first is LIPOchip microarray analysis for the detection of the most frequent Spanish point mutations in the LDLR gene and in the APOB exon 26, as well as CNVs in LDLR. When the LIPOchip microarray yielded a negative result (no mutation was found), the LDLR, APOB (binding domain) and PCSK9 gene coding sequences, exon-intron boundaries, and short proximal intronic sequences were sequenced with a GS Junior system (Roche Diagnostics Corporation, Basel, Switzerland).25
Statistical analysisData are expressed as mean±standard deviation for continuous variables. Categorical variables are expressed as percentages and frequencies. The Student t test was performed to assess differences between 2 means. The chi-square test or the Fisher exact test wase used to evaluate the degree of association among categorical variables. Logistic regression analysis was applied and odds ratio (OR) and 95% confidence intervals (95%CI) were calculated to assess whether, after adjustment for the remaining variables influencing the presence of CVD, the existence of an impaired glucose metabolism (including T2DM diagnosis, fasting blood glucose and HbA1c) was associated with the presence of CVD in patients with HeFH. The initial model was unadjusted; the second model was adjusted for age and male sex; the third model for age, male sex, smoking, and HDL cholesterol concentrations and finally, the last model was adjusted for age, male sex, smoking, HDL cholesterol concentrations, hypertension, and body mass index. A 2-sided P value <.05 was considered statistically significant. Analyses were performed with SPSS (version 19.0 for Windows; SPSS, Chicago, IL, United States).
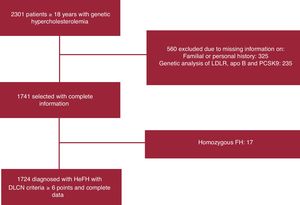
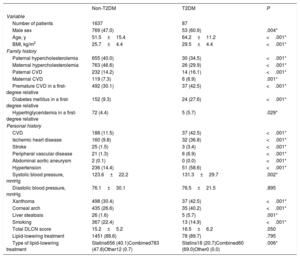
RESULTSOf the 2301 patients with primary hypercholesterolemia included in the registry, 1724 with a probable or definite diagnosis according to the Dutch Lipid Clinic Network score were finally included (figure 1). The mean age of the study cohort was 52.2±15.4 years, and 822 (47.7%) were male, with a mean body mass index of 25.9±4.5kg/m2. Eighty-seven of the 1724 (5%) included in the final analysis met the criteria for T2DM (table 1).
Study flowchart of the patients finally included in the analysis. apo, apolipoprotein; DLCN, Dutch Lipid Clinic Network; FH, familial hypercholesterolemia; HeFH, heterozygous familial hypercholesterolemia; LDLR, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type-9.
Clinical characteristics of patients with heterozygous familial hypercholesterolemia included in the study
| Non-T2DM | T2DM | P | |
|---|---|---|---|
| Variable | |||
| Number of patients | 1637 | 87 | |
| Male sex | 769 (47.0) | 53 (60.9) | .004* |
| Age, y | 51.5±15.4 | 64.2±11.2 | <.001* |
| BMI, kg/m2 | 25.7±4.4 | 29.5±4.4 | <.001* |
| Family history | |||
| Paternal hypercholesterolemia | 655 (40.0) | 30 (34.5) | <.001* |
| Maternal hypercholesterolemia | 763 (46.6) | 26 (29.9) | <.001* |
| Paternal CVD | 232 (14.2) | 14 (16.1) | <.001* |
| Maternal CVD | 119 (7.3) | 6 (6.9) | .001* |
| Premature CVD in a first-degree relative | 492 (30.1) | 37 (42.5) | <.001* |
| Diabetes mellitus in a first-degree relative | 152 (9.3) | 24 (27.6) | <.001* |
| Hypertriglyceridemia in a first-degree relative | 72 (4.4) | 5 (5.7) | .029* |
| Personal history | |||
| CVD | 188 (11.5) | 37 (42.5) | <.001* |
| Ischemic heart disease | 160 (9.8) | 32 (36.8) | <.001* |
| Stroke | 25 (1.5) | 3 (3.4) | <.001* |
| Peripheral vascular disease | 21 (1.3) | 6 (6.9) | <.001* |
| Abdominal aortic aneurysm | 2 (0.1) | 0 (0.0) | <.001* |
| Hypertension | 236 (14.4) | 51 (58.6) | <.001* |
| Systolic blood pressure, mmHg | 123.6±22.2 | 131.3±29.7 | .002* |
| Diastolic blood pressure, mmHg | 76.1±30.1 | 76.5±21.5 | .895 |
| Xanthoma | 498 (30.4) | 37 (42.5) | <.001* |
| Corneal arch | 435 (26.6) | 35 (40.2) | <.001* |
| Liver steatosis | 26 (1.6) | 5 (5.7) | .001* |
| Smoking | 367 (22.4) | 13 (14.9) | <.001* |
| Total DLCN score | 15.2±5.2 | 16.5±6.2 | .050 |
| Lipid-lowering treatment | 1451 (88.6) | 78 (89.7) | .795 |
| Type of lipid-lowering treatment | Statins656 (40.1)Combined783 (47.8)Other12 (0.7) | Statins18 (20.7)Combined60 (69.0)Other0 (0.0) | .006* |
BMI, body mass index; CVD, cardiovascular disease; DLCN, Dutch Lipid Clinic Network; T2DM, type 2 diabetes mellitus.
Data are expressed as No. (%) or mean±standard deviation.
Paternal and maternal hypercholesterolemia rates were higher in patients with a normal glucose profile compared with those with T2DM. However, rates of premature CVD disease and DM in a first-degree relative were higher in the HeFH group with T2DM.
Compared with HeFH without T2DM, those with T2DM were more frequently male (60.9% vs 47.0%; P=.004), were older (64.2±11.2 years vs 51.5±15.4 years; P <.001), and had a higher body mass index (29.5±4.4kg/m2 vs 25.7±4.4kg/m2; P <.001).
These patients also had higher rates of CVD (42.5% vs 11.5%; P <.001) regardless of the involved area (coronary, cerebrovascular or lower extremities) and higher hypertension rates (58.6% vs 14.4%; P <.001). T2DM patients also more frequently had xanthomas (42.5% vs 30.4%; P <.001) and corneal arch (40.2% vs 26.6%; P <.001) compared with those without DM. The rate of abdominal aortic aneurysms was lower in HeFH patients with T2DM than in those with a normal glucose profile (0.0% vs 0.1%; P <.001). The remaining clinical characteristics are shown in table 1.
Biochemical parametersThe cardiovascular event was prior to the start of lipid-lowering treatment in 140 cases; of these, 21 (15%) had T2DM. The biochemical parameters of HeFH patients with and without lipid-lowering treatment were also analyzed.
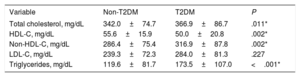
Based on biochemical results without lipid-lowering treatment, patients with T2DM and HeFH had a less favorable lipid profile, with higher total (366.9±86.7mg/dL vs 342.0±74.7mg/dL; P=.011) and non-HDL (316.9±87.8mg/dL vs 286.4±75.4mg/dL; P=.002) cholesterol levels. Furthermore, HDL cholesterol concentrations were lower (50.0±20.8mg/dL vs 55.6±15.9mg/dL; P=.002) and triglycerides were higher (173.5±107.0mg/dL vs 119.6±81.7mg/dL; P <.001) than in the group with normoglycemia. No differences were found in LDL cholesterol levels between groups (table 2).
Lipid profile without lipid-lowering treatment in patients with heterozygous familial hypercholesterolemia included in the study
| Variable | Non-T2DM | T2DM | P |
|---|---|---|---|
| Total cholesterol, mg/dL | 342.0±74.7 | 366.9±86.7 | .011* |
| HDL-C, mg/dL | 55.6±15.9 | 50.0±20.8 | .002* |
| Non-HDL-C, mg/dL | 286.4±75.4 | 316.9±87.8 | .002* |
| LDL-C, mg/dL | 239.3±72.3 | 284.0±81.3 | .227 |
| Triglycerides, mg/dL | 119.6±81.7 | 173.5±107.0 | <.001* |
HDL-C, high-density lipopoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; T2DM, type 2 diabetes mellitus.
Data are expressed as mean±standard deviation.
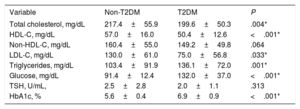
Blood examination results of patients on lipid-lowering treatment were also evaluated (table 3). In this scenario, total cholesterol (217.4±55.9mg/dL vs 199.6±50.3mg/dL; P=.004) and LDL cholesterol (130.0±61.0mg/dL vs 75.0±56.8mg/dL; P=.033) levels were significantly higher in the non-T2DM group than in patients with T2DM.
Current laboratory parameters of patients with heterozygous familial hypercholesterolemia included in the study
| Variable | Non-T2DM | T2DM | P |
|---|---|---|---|
| Total cholesterol, mg/dL | 217.4±55.9 | 199.6±50.3 | .004* |
| HDL-C, mg/dL | 57.0±16.0 | 50.4±12.6 | <.001* |
| Non-HDL-C, mg/dL | 160.4±55.0 | 149.2±49.8 | .064 |
| LDL-C, mg/dL | 130.0±61.0 | 75.0±56.8 | .033* |
| Triglycerides, mg/dL | 103.4±91.9 | 136.1±72.0 | .001* |
| Glucose, mg/dL | 91.4±12.4 | 132.0±37.0 | <.001* |
| TSH, U/mL, | 2.5±2.8 | 2.0±1.1 | .313 |
| HbA1c, % | 5.6±0.4 | 6.9±0.9 | <.001* |
HDL-C, high-density lipoprotein cholesterol; HbA1c, glycosylated hemoglobin; LDL-C, low-density lipoprotein cholesterol; T2DM, type 2 diabetes mellitus; TSH, thyroid-stimulating hormone.
Data are expressed as mean±standard deviation.
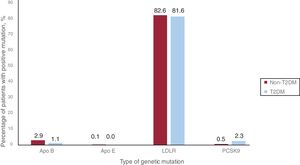
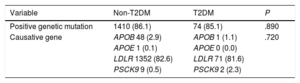
Seventy-four (85.1%) and 1410 (86.1%) patients with and without T2DM, respectively, had a pathogenic mutation related to HeFH (P=.890). The LDLR mutation was the most frequent, being present in 81.6% and 82.6% of patients with and without T2DM, respectively. The remaining results of the genetic study of the included patients are detailed in table 4 and figure 2.
Genetic analysis of included patients
| Variable | Non-T2DM | T2DM | P |
|---|---|---|---|
| Positive genetic mutation | 1410 (86.1) | 74 (85.1) | .890 |
| Causative gene | APOB 48 (2.9) | APOB 1 (1.1) | .720 |
| APOE 1 (0.1) | APOE 0 (0.0) | ||
| LDLR 1352 (82.6) | LDLR 71 (81.6) | ||
| PSCK9 9 (0.5) | PSCK9 2 (2.3) |
APO, apolipoprotein; LDLR, low-density lipoprotein receptor; PCSK9, proprotein convertase subtilisin/kexin type-9; T2DM, type 2 diabetes mellitus.
Data are expressed as No. (%)
The association between impaired glucose metabolism and CVD in patients with HeFH was also evaluated (table 5). After adjustment for several risk factors such as age, male sex, smoking, HDL cholesterol levels, hypertension, and body mass index, a significant association was found between T2DM and CVD (OR, 2.01; 95%CI, 1.18-3.43; P=.010).
Logistic regression analysis evaluating the association between impaired glucose metabolism and cardiovascular disease in patients with heterozygous familial hypercholesterolemia
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| T2DMOR (95%CI) | 5.72 (3.64-8.99)P <.001* | 2.97 (1.82-4.87)P <.001* | 2.65 (1.59-4.44)P <.001* | 2.01 (1.18-3.43)P=.010* |
| Fasting blood glucoseOR (95%CI) | 1.03 (1.02-1.04)P <.001* | 1.01 (1.00-1.03)P=.025* | 1.01 (1.00-1.03)P=.027* | 1.01 (0.99-1.02)P=.105 |
| HbA1cOR (95%CI) | 2.30 (1.45-3.64)P <.001* | 1.78 (1.10-2.88)P=.018* | 1.83 (1.11-3.01)P=.018* | 1.52 (0.92-2.53)P=.104 |
95%CI, 95% confidence interval; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; OR, odds ratio; T2DM, type 2 diabetes mellitus.
Model 1: Unadjusted.
Model 2: Adjusted for age and male sex.
Model 3: Adjusted for age, male sex, smoking, and HDL cholesterol concentrations.
Model 4: Adjusted for age, male sex, smoking, HDL cholesterol concentrations, hypertension and body mass index.
Recent clinical evidence has demonstrated that CVD among patients with HeFH has substantially improved with current management, with the prevalence of CVD being one third of that reported before the statin era.26 However, CVD in HeFH is extremely heterogeneous. Although specific HeFH risk factors such as the type of mutation and the presence of tendon xanthomas have been previously described,27 classic cardiovascular risk factors also appear to play a role in explaining differences in the presentation of CVD in the FH population.7
In the present study, HeFH patients with T2DM had higher rates of hypertension and CVD, including ischemic heart disease, stroke, and peripheral vascular disease, but a lower rate of abdominal aortic aneurysm. Moreover, they also had a less favorable lipid profile, with higher total cholesterol, non-HDL cholesterol, and triglyceride levels and lower HDL cholesterol levels. Finally, the frequency and type of genetic mutation was similar in both groups, with mutations in LDLR being the most frequently encountered mutation in this cohort.
T2DM is an established cardiovascular risk factor associated with an increased risk of coronary, cerebral and peripheral vascular disease.28 Thus, it is not surprising that, in the present cohort, the CVD rate was higher in patients with impaired glucose metabolism than in those without T2DM. Concurring with these results, Yanagi et al.29 also found an 87% and 59% increase in the prevalence of coronary artery disease in patients with FH with impaired glucose tolerance and T2DM compared with the 43% in patients with a normal glucose profile.
Furthermore, in agreement with the present study, recent data from the Canadian FH cohort suggested that diabetic FH patients represent a high cardiovascular risk population owing to the presence of concomitant cardio-metabolic risk factors.17 In agreement with previous studies,30,31 other factors associated with insulin resistance such as older age, increased body mass index and high blood pressure were also more frequent in the T2DM group in the present study, probably also playing a role in the higher cardiovascular risk in this specific population.
Since atherogenic dyslipidemia is the characteristic lipid abnormality in T2DM, it seems reasonable that, in the present cohort, HDL cholesterol levels were lower and triglyceride levels were higher in the group of patients with T2DM. Nevertheless, LDL cholesterol levels were comparable between groups, also in agreement with other reports.29,32 Moreover, concurring with the present results, a previous study published by our group, which included 354 patients with probable HeFH and 1378 with definite HeFH, found LDL cholesterol concentration not to be a risk factor associated with the prevalence of T2DM.33 Similarly, other prospective studies also failed to find a direct relationship between LDL cholesterol levels and increased diabetes risk.34,35
As for the lipid profile after initiation of lipid-lowering treatment, as mentioned previously in the results section, total and LDL cholesterol levels were significantly higher in the non-T2DM group than in patients with T2DM. This can be probably explained by the greater percentage of lipid-lowering treatment use in the group of patients with an impaired glucose metabolism.
Around 85% of patients both with and without T2DM had a causative genetic mutation of HeFH; however, no differences between groups were observed in the frequency or distribution of the type of affected gene. These results agree with those of a study conducted with the same registry of patients that found no differences in the prevalence of T2DM according to the presence of certain gene mutations causing HeFH, including LDLR, APOB and PCSK9.33
However, unlike the previously described results, other studies have found a possible association between HeFH causative mutations and modifications in the risk of showing alterations in glycemic metabolism. For instance, Besseling et al.10 found a lower prevalence of T2DM in patients with an LDLR-negative mutation with a dose-dependent association compared with those with a defective LDLR or APOB mutation, thus suggesting a possible relationship between the severity of the genetic alteration and T2DM protection. Similarly, Saavedra et al.36 concluded that the InsLEU variant of the PCSK9 gene was associated not only with a lower risk of coronary events but also with an increased occurrence of pre-DM and DM. In summary, HeFH causative mutations have been related to both an increase and a decrease in altered glycemic metabolism, although the present study found no association between the presence of T2DM and certain genetic mutations. Hence, owing to the conflicting results in this field, further studies will be needed to confirm a possible relationship between the type of HeFH causative mutation and an altered glycemic profile.
Finally, and in line with the previously mentioned results, a positive association was found between impaired glucose metabolism (including T2DM, fasting blood glucose levels and HbA1c) and CVD in HeFH patients, thus confirming once again the close relationship between them.
The present study has some limitations. First, because it was an observational study with a cross-sectional design, no causal relationship could be inferred from the results. The number of patients with T2DM and HeFH was relatively small in comparison with the general Spanish population (5% vs 13.8% in> 18 years and 29.8% in the age range 65-71 years),37 thus concurring with the possible protective effect of HeFH on diabetes risk. As for the population included, participants were extracted from the national registry of a Mediterranean country where lower coronary heart disease rates are expected compared with other populations worldwide, albeit with a high prevalence of T2DM.37 Furthermore, all participants included in this study were extracted from the dyslipidemia registry of the Spanish Arteriosclerosis Society. These patients are therefore treated and followed up at specialized lipid units following a standardized protocol and, consequently, the present results cannot be extrapolated to the whole Spanish population.
CONCLUSIONSIn the present study, HeFH patients with T2DM had higher rates of hypertension and CVD, together with a less favorable lipid profile. A positive association was confirmed between impaired glucose metabolism and CVD in HeFH patients. No differences were found between groups in the presence or distribution of the different causative genetic mutations of HeFH. Future studies in this field will help to identify possible differences between patients diagnosed with HeFH with and without impaired glucose metabolism.
FundingThis article has not received any funding source.
CONFLICTS OF INTERESTJ.F. Ascaso reports personal fees from Sanofi, Mylan, Ferrer, and Novo Nordisk, outside the submitted work; J. Pedro-Botet reports nonfinancial support from Astra-Zeneca, Esteve, Merck, Mylan, and Sanofi-Aventis, outside the submitted work; X. Pintó reports grants from FIS-ISCIII and CIBER-ISCIII, personal fees from Amgen, Abbott, Sanofi, Lacer, Rubió, and Esteve, outside the submitted work; P. Valdivielso reports personal fees from Amgen, Sanofi, and MSD, grants and personal fees from Ferrer, and personal fees from Esteve, outside the submitted work; other authors report no conflicts of interest related to this work.
- -
Patients with HeFH are at increased cardiovascular risk.
- -
Patients with HeFH have a lower T2DM rate.
- -
T2DM has not always been described as a cardiovascular risk factor in these patients.
- -
Patients with HeFH and T2DM have a higher CVD risk.
- -
Patients with HeFH and T2DM have a less favorable lipid profile.
- -
No differences were observed regarding specific genetic mutations in HeFH patients with or without T2DM.
- -
T2DM is associated with the presence of CVD in these patients.
We thank the personnel of Spanish Lipid Clinics for inclusion of cases in the dyslipidemia registry of the Spanish Arteriosclerosis Society, and Miss Christine O’Hara for review of the English version of the manuscript.