Circulating ST2 is the soluble isoform of the interleukin (IL) 1 receptor family and was first described in 1989 in relation to immune-mediated inflammatory responses.1 This isoform caught the attention of the cardiovascular community in 2001, when a study of 7000 gene transcripts of known function found that the ST2 gene was strongly induced by mechanical strain in cardiac myocytes.2
A recent article by Arrieta et al.3 in Revista Española de Cardiología provides new insights that support a shift in our understanding of the role of circulating ST2 in cardiovascular disease. In this new perspective, ST2 is seen not only as a prognostic biomarker but also as a potential pathogenic mediator involved in disease development, in this case, myocardial fibrosis and adverse ventricular modeling in the setting of aortic stenosis. In their groundbreaking work published in 2002, Weinberg et al.2 described an increase in circulating ST2 levels in the first few hours following myocardial infarction. Shortly afterward, the same group identified ST2 as a novel biomarker of heart failure.4 In the 20 years since that discovery, numerous authors have reported associations between increased circulating ST2 levels and a higher risk of clinical complications, particularly mortality.5 The methods used to measure ST2 have also changed over the years, from a wide variety of manual immunoassays to quality-assured automated laboratory tests and even rapid point-of-care tests. In 2013, the US Clinical Practice Guidelines on the Management of Heart Failure recommended measuring soluble ST2 levels to assess myocardial fibrosis risk.6 Use of this biomarker in clinical practice in Europe, however, remains low.
Our group became interested in soluble ST2 from the outset, and produced novel evidence of its association with worse outcomes in heart failure. Two of our findings were particularly relevant: first, ST2 provided complementary prognostic information to natriuretic peptides and troponins7,8 and second, the isoform was a potent predictor of mortality, specifically sudden cardiac death.9 Notably, other studies have shown associations between soluble ST2 and adverse myocardial remodeling, particularly after acute myocardial infarction.10 The ability of ST2 to provide complementary information to other biomarkers should not be taken to indicate that it is solely a biomarker of risk, but rather that this isoform could be involved in a distinct pathophysiological process and probably plays a pathogenic role. Support for this role is provided by the strong association between ST2 and mortality and its predictive value in a range of diseases and clinical contexts, all in the setting of cardiovascular disease and progressive myocardial injury and dysfunction. Cumulative evidence demonstrates that higher circulating ST2 levels are predictive of increased risk, not only in patients with existing heart failure,11 but also in patients with other heart conditions—particularly myocardial infarction—and even nonheart-related conditions that can cause myocardial injury or increase myocardial risk, such as acute respiratory distress and, more recently, COVID-19.5,12
The findings reported by Arrieta et al.3 are interesting for a number of reasons. First, they add to the evidence supporting the association between higher circulating ST2 levels and adverse myocardial remodeling with greater ventricular dysfunction. This association has been well established over the years, both after acute myocardial infarction and in patients with heart failure, and has even been included in risk stratification models for adverse remodeling.10,13 Second, the findings of Arrieta et al. provide evidence of a role for ST2 in aortic stenosis, a valvular heart disease that carries a risk of progressive myocardial injury and dysfunction. The most relevant finding, however, is the link between higher circulating ST2 levels and the degree of myocardial fibrosis assessed by cardiac magnetic resonance imaging. These levels play a clear pathogenic role in adverse ventricular remodeling and risk of ventricular dysfunction and ventricular arrhythmias, and there are currently no effective ST2-lowering treatments. In this regard, a striking finding in the study by Arrieta et al. is the consistent association between high soluble ST2 levels (≥ 28.2 ng/mL) and the presence of fibrosis, which further strengthens evidence of the link. The authors also demonstrated a stronger pathogenic association in men, who showed greater fibrosis and adverse remodeling. This could be an artifact of the inherent limitations of the cross-sectional design of the study, its small sample size, and confounder imbalance, but at the very least this finding supports the need for more sex-stratified studies, not only of ST2 but also of many other biological systems.
Recent research by our group, whose pioneering work showed an association between ST2 and increased risk, has focused on elucidating the mechanisms of ST2 modulation, with the hypothesis that, if ST2 is a pathogenic mediator, then, like many other elements in the cardiovascular system, it should be considered a therapeutic target.14 In this regard, we have demonstrated that the myocardium is not the primary source of circulating soluble ST2, showing instead that the lungs, together with endothelial and inflammatory cells, play an essential role in its production.15–17 Furthermore, the release of circulating ST2 should be interpreted as an inflammatory, immune-mediated response.18 The main limitation of the work by Arrieta et al.1 is that, as a cross-sectional study, it can only describe associations, and excludes the possibility of drawing causal inferences. Causality, however, can be indirectly inferred from the cumulative body of evidence and the experimental studies conducted over the past 20 years,5,14,19 whose findings increasingly point to a role for ST2 in the development of fibrosis and hypertrophy, with progressive adverse remodeling and myocardial dysfunction.
One of the remaining challenges in cardiovascular medicine is to identify therapeutic applications for the information gathered on the multiple risk biomarkers discovered over the years. We recently saw that at least some of the benefits of neprilysin inhibition are linked to an enhancement of the favorable effects of natriuretic peptides (which, as a result of inhibition, are not eliminated). The pathogenic effect of circulating or soluble ST2 is probably linked to inhibition of the cardioprotective interaction between IL-33 and the membrane-bound ST2 receptor, ST2L, whose signaling prevents hypertrophy, inflammation, and fibrosis.19,20 Over the years, experimental models have amply shown the protective effect of ST2L on cardiomyocytes and its interaction with IL-33.14,21,22 Conversely, research has also shown the detrimental effects of its genetic deletion.23 In this same model, addition of the soluble ST2 isoform resulted in a loss of cardioprotection due to ineffective IL-33 sequestration.24 There is also evidence that at least some of the benefits of drugs such as mineralocorticoid receptor agonists are probably derived from favorable modulation of ST2 signaling.19,22
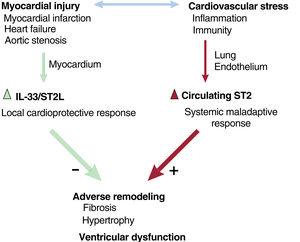
We can therefore state that cardiac injury induces 2 responses: a local cardioprotective response in the myocardium and a systemic, maladaptive, inflammatory response that may lead to the loss of this favorable local response (figure 1). This raises the question of how best to strengthen this cardioprotective signaling pathway. The cumulative evidence would seem to suggest that the answer is to prevent the production of soluble or circulating ST2, as increased levels of this isoform are associated with worse outcomes in a range of cardiovascular diseases involving myocardial injury, including severe aortic stenosis.3,5,20 Our group recently identified a number of key elements modulating the expression of circulating levels of the soluble ST2 isoform.25
Interactions between ST2 and the cardiovascular system. Myocardial injury and cardiovascular stress induce a local cardioprotective response (ST2L/IL-33) and a systemic maladaptive response that results in increased circulating levels of soluble ST2, causing myocardial inhibition of favorable signaling pathways and greater adverse remodeling, hypertrophy, fibrosis, and cardiac dysfunction. IL-33, interleukin 33; ST2L, membrane-bound ST2 isoform.
The findings described in the study by Arrieta et al.1 support the theory that ST2 is not simply a useful biomarker in risk stratification, but is also a pathogenic mediator that leads to cardiac dysfunction and remodeling, with myocardial hypertrophy and fibrosis. There is therefore a need for treatments to reduce ST2 circulation, thus achieving a cardioprotective effect.
FundingA. Lax is a “Ramón y Cajal” researcher at the Department of Medicine at the University of Murcia (RYC2019-027635-I) and M.C. Asensio-López is a “Juan de la Cierva” researcher at the Hematovascular Pathophysiologic Laboratory at Centro Nacional de Investigaciones Cardiovasculares (FJC2020-042841-I). Support was received through grants from Instituto de Salud Carlos III (PI19/00519; PI14/01637).
Conflicts of InterestThe authors have registered a patent related to ST2 modulation (PCT/EP2021/065521).